LAG3 regulates antibody responses in a murine model of kidney transplantation
- PMID: 40359031
- PMCID: PMC12208549
- DOI: 10.1172/JCI172988
LAG3 regulates antibody responses in a murine model of kidney transplantation
Abstract
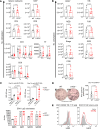
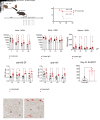
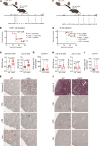
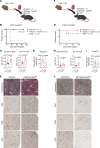
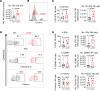
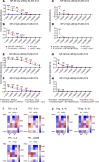
Lymphocyte activation gene 3 (LAG3) is a coinhibitory receptor expressed by various immune cells. Although the immunomodulatory potential of LAG3 is being explored in cancer and autoimmunity, there is no information on its role after organ transplantation. Our study investigated the functions of LAG3 in a mouse model of renal allograft rejection. LAG3-/- recipients rapidly rejected MHC-mismatched renal allografts that were spontaneously accepted by WT recipients, with graft histology characteristic of antibody-mediated rejection. Depletion of recipient B cells but not CD8+ T cells significantly extended kidney allograft survival in LAG3-/- recipients. Treatment of WT recipients with an antagonistic LAG3 antibody enhanced anti-donor immune responses and induced kidney damage associated with chronic rejection. The studies of conditional LAG3-/- recipients and mixed bone marrow chimeras demonstrated that LAG3 expression on either T or B cells is sufficient to regulate anti-donor humoral immunity but not to induce acute allograft rejection. The numbers and proinflammatory functions of graft-infiltrating NK cells were markedly increased in LAG3-/- recipients, suggesting that LAG3 also regulates the effector stage of antibody-mediated rejection. These findings identified LAG3 as a regulator of immune responses to kidney allografts and a potential therapeutic target for antibody-mediated rejection prevention and treatment.
Keywords: Adaptive immunity; Immunology; Transplantation.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

