Activation of intestinal endogenous retroviruses by alcohol exacerbates liver disease
- PMID: 40359032
- PMCID: PMC12208555
- DOI: 10.1172/JCI188541
Activation of intestinal endogenous retroviruses by alcohol exacerbates liver disease
Abstract
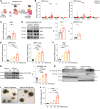
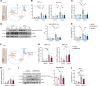
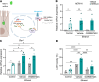
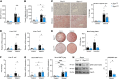
Alcohol-associated liver disease represents a significant global health challenge, with gut microbial dysbiosis and bacterial translocation playing a critical role in its pathogenesis. Patients with alcohol-associated hepatitis had increased fecal abundance of mammalian viruses, including retroviruses. This study investigated the role of endogenous retroviruses (ERVs) in the development of alcohol-associated liver disease. Transcriptomic analysis of duodenal and liver biopsies revealed increased expression of several human ERVs, including HERV-K and HERV-H, in patients with alcohol-associated liver disease compared with individuals acting as controls. Chronic-binge ethanol feeding markedly induced ERV abundance in intestinal epithelial cells but not the livers of mice. Ethanol increased ERV expression and activated the Z-DNA binding protein 1 (Zbp1)-mixed lineage kinase domain-like pseudokinase (Mlkl) signaling pathways to induce necroptosis in intestinal epithelial cells. Antiretroviral treatment reduced ethanol-induced intestinal ERV expression, stabilized the gut barrier, and decreased liver disease in microbiota-humanized mice. Furthermore, mice with an intestine-specific deletion of Zbp1 were protected against bacterial translocation and ethanol-induced steatohepatitis. These findings indicate that ethanol exploits this pathway by inducing ERVs and promoting innate immune responses, which results in the death of intestinal epithelial cells, gut barrier dysfunction, and liver disease. Targeting the ERV/Zbp1 pathway may offer new therapies for patients with alcohol-associated liver disease.
Keywords: Gastroenterology; Hepatology; Microbiome; Molecular biology.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

