The gut microbiome controls reactive astrocytosis during Aβ amyloidosis via propionate-mediated regulation of IL-17
- PMID: 40359034
- PMCID: PMC12208551
- DOI: 10.1172/JCI180826
The gut microbiome controls reactive astrocytosis during Aβ amyloidosis via propionate-mediated regulation of IL-17
Abstract
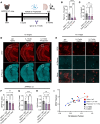
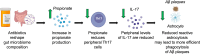
Accumulating evidence implicates the gut microbiome (GMB) in the pathogenesis and progression of Alzheimer's disease (AD). We recently showed that the GMB regulates reactive astrocytosis and Aβ plaque accumulation in a male APPPS1-21 AD mouse model. Yet, the mechanism(s) by which GMB perturbation alters reactive astrocytosis in a manner that reduces Aβ deposition remain unknown. Here, we performed metabolomics on plasma from mice treated with antibiotics (ABX) and identified a significant increase in plasma propionate, a gut-derived short-chain fatty acid, only in male mice. Administration of sodium propionate reduced reactive astrocytosis and Aβ plaques in APPPS1-21 mice, phenocopying the ABX-induced phenotype. Astrocyte-specific RNA-Seq on ABX- and propionate-treated mice showed reduced expression of proinflammatory and increased expression of neurotrophic genes. Next, we performed flow cytometry experiments, in which we found that ABX and propionate decreased peripheral RAR-related orphan receptor-γ+ (Rorγt+) CD4+ (Th17) cells and IL-17 secretion, which positively correlated with reactive astrocytosis. Last, using an IL-17 mAb to deplete IL-17, we found that propionate reduced reactive astrocytosis and Aβ plaques in an IL-17-dependent manner. Together, these results suggest that gut-derived propionate regulates reactive astrocytosis and Aβ amyloidosis by decreasing peripheral Th17 cells and IL-17 release. Thus, propionate treatment or strategies boosting propionate production may represent novel therapeutic strategies for the treatment of AD.
Keywords: Alzheimer disease; Cellular immune response; Immunology; Microbiology; Neuroscience.
Conflict of interest statement
Figures






Comment in
- Thwarting amyloidosis: IL-17 as a disease modifier along the gut/brain axis doi: 10.1172/JCI194443
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

