State of the Art and Consensus Statements by Healthcare Providers, Patients, and Caregivers on Continuous Glucose Monitoring in Liver Glycogen Storage Diseases
- PMID: 40360288
- PMCID: PMC12074895
- DOI: 10.1002/jimd.70040
State of the Art and Consensus Statements by Healthcare Providers, Patients, and Caregivers on Continuous Glucose Monitoring in Liver Glycogen Storage Diseases
Abstract
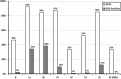
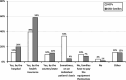
Continuous glucose monitoring (CGM) is increasingly used although not officially registered for the management of people living with liver glycogen storage diseases (GSDs). The aims of this study were twofold: (a) to investigate the current experiences of healthcare providers (HCPs), patients, and caregivers using CGM to monitor glucose concentrations in liver GSDs, and (b) to formulate consensus statements. Two web-based questionnaires were distributed, one for HCPs and one for patients and/or their caregivers. The questionnaires collected data on demographics and epidemiology, current use of CGM, and opinions and statements about CGM in GSDs. For the statements, respondents rated their agreement on a 5-point Likert scale, and the consensus level was set at 75%. One Hundred Fourteen HCPs (including 87 physicians and 26 dietitians) from 28 countries responded, representing care of approximately 3800 liver GSD patients. Additionally, 148 GSD patients and/or their caregivers from 21 countries responded, mainly representing GSD Ia (n = 50), GSD Ib (n = 56), GSD III (n = 14), and GSD IX (n = 18). The median age to consider starting to use CGM was 6 and 2 months for HCPs and GSD families, respectively. Out of 16 statements common to the two questionnaires, HCPs and patients/caregivers reached consensus on 12 statements in both groups. Use of CGM is considered standard of care by both HCPs and GSD families, but reimbursement of CGM devices is challenging. Compared to diabetes mellitus, CGM should be applied differently in liver GSDs. Consensus guidelines are warranted on the use of CGM in liver GSDs, both in routine healthcare and in clinical trials.
Keywords: glycemic control; management; rare disease; survey.
© 2025 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Terry G. J. Derks: There are confidentiality agreements with third parties. In the past 36 months, there have been consultation agreements (with Danone S.A., Ultragenyx Pharmaceutical Inc., Moderna Inc., and Beam Therapeutics Inc.), contracts for financial research support for investigator‐initiated research (NCT04311307) and sponsor‐initiated research (NCT03517085, NCT03970278, NCT05139316, and NCT05196165), honoraria for lectures or presentations (by MEDTalks, Prelum, and Danone S.A.), and participations in a Data Safety Monitoring Board (NCT05095727) and Advisory Boards (Ultragenyx Pharmaceutical Inc., Moderna Inc., and Beam Therapeutics Inc.). For all private‐public relationships, all contracts are via UMCG Contract Research Desk and all payments are to UMCG. Sarah C. Grünert: She has received honoraria for educational lectures from Vitaflo GmbH and Ultragenyx Pharmaceutical Inc. as well as for the creation of patient information material for Danone Deutschland GmBH, and received support for attending metabolic expert meetings from Nutricia Metabolics GmbH. She participated in an advisory board for Ultragenyx Pharmaceutical Inc. Ruben J. Overduin: There are confidentiality agreements with third parties. There is a consultation agreement (with Ultragenyx Pharmaceutical Inc.). For all private‐public relationships, all contracts are via UMCG Contract Research Desk and all payments are to UMCG. Alessandro Rossi: There are confidentiality agreements with third parties. In the past 36 months, there have been consultation agreements or honoraria for lectures or presentations with Nestlé, Danone S.A., and Ultragenyx Pharmaceutical Inc.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

