Noncatalyzed Intramolecular B-N and B-O Cross-Coupling of "Inert" Carboranes Lead to the Formation of an Unusual Oxoborane, via Reversible Cluster C-B Bond Scission
- PMID: 40360424
- PMCID: PMC12123610
- DOI: 10.1021/jacs.5c01106
Noncatalyzed Intramolecular B-N and B-O Cross-Coupling of "Inert" Carboranes Lead to the Formation of an Unusual Oxoborane, via Reversible Cluster C-B Bond Scission
Abstract
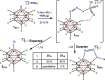
Polyhalogenated closo-12-vertex carborane anions are thought to be inert species incapable of participating in direct B-X substitution reactions. Here, we show that this is not true and that such species can be easily coaxed into intramolecular cross-coupling cyclizations without the need for a catalyst. When cage C-tethered O and N-heteroallylic anions are generated, a variety of cyclized products can be formed in high yield under mild conditions. Additionally, we show that even C-tethered neutral nucleophiles, such as the pyridine moiety, undergo facile B-X substitution chemistry and these reactions are not dependent on the countercation. Serendipitously, we also found that when these cyclizations are attempted with acetamide derivatives, an unprecedented cluster C-B bond scission reaction occurs, producing an unprecedented oxoborane stabilized by multicentered bonding. Amazingly this molecule can be protonated, leading to reformation of the C-B bond and cluster reorganization, and this process is reversible.
Figures




References
-
- Roshanzadeh A., Medeiros H. C. D., Herrera C. K., Malhado C., Tomich A. W., Fisher S. P., Lovera S. O., Bates M., Lavallo V., Lunt R. R.. et al. Next-Generation Photosensitizers: Cyanine-Carborane Salts for Superior Photodynamic Therapy of Metastatic Cancer. Angew. Chem., Int. Ed. 2025;64(9):e202419759. doi: 10.1002/anie.202419759. - DOI - PMC - PubMed
-
- Jo̷rgensen M., Shea P. T., Tomich A. W., Varley J. B., Bercx M., Lovera S., Černý R., Zhou W., Udovic T. J., Lavallo V.. et al. Understanding Superionic Conductivity in Lithium and Sodium Salts of Weakly Coordinating Closo-Hexahalocarbaborate Anions. Chem. Mater. 2020;32(4):1475–1487. doi: 10.1021/acs.chemmater.9b04383. - DOI
LinkOut - more resources
Full Text Sources

