Sexual Dimorphism in Levodopa-Induced Dyskinesia Following Parkinson's Disease: Uncharted Territory
- PMID: 40360439
- PMCID: PMC12075048
- DOI: 10.1111/ejn.70144
Sexual Dimorphism in Levodopa-Induced Dyskinesia Following Parkinson's Disease: Uncharted Territory
Abstract
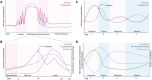
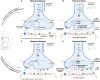
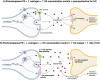
Sexual dimorphism is well-documented in Parkinson's disease (PD); however, when it comes to levodopa-induced dyskinesia (LID), epidemiological and clinical findings are scarce. This is an oversight because recent studies show significant correlations between LID risk and female sex. Estrogen strongly impacts neuronal function, affecting cognitive tasks such as movement, object recognition, and reward. In movement pathways, estrogen increases dopamine synthesis, transmission, and regulation, resulting in neuroprotection for PD in women. However, following menopause, PD prevalence, symptom severity, and LID risk increase for women. Consequently, early to mid-life estrogen state is neuroprotective, but later in life becomes a risk factor for PD and LID. This review explores estrogen's action in the brain, specifically within the dopamine system. Sexual dimorphism is described for the prevalence and onset of PD and LID. We examine the cellular basis of estrogen's role in sexual dimorphism and integrate these ideas to hypothesize why the risk for LID is higher for women, than men, with PD. Lastly, this review proposes that women with PD need their symptoms to be considered and managed differently to males. Treatment of women with PD should be based on their menopausal stage, as estrogen may be masking, exacerbating, or complicating symptoms. Importantly, we present these concepts to stimulate discussion among clinical and bench scientists so that key experiments can be conducted to examine the mechanisms underlying LID, so they can be prevented to improve the quality of life for women and men living with PD in the future.
Keywords: Parkinson; basal ganglia; estrogen; levodopa‐induced dyskinesia; menopause; sexual dimorphism; striatum.
© 2025 The Author(s). European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ahlskog, E. , and Muenter M.. 2001. “Frequency of Levodopa‐Related Dyskinesias and Motor Fluctuations as Estimated From the Cumulative Literature.” Movement Disorders 16, no. 3: 448–458. - PubMed
-
- Almey, A. , Cannell E., Bertram K., Filardo E., Milner T. A., and Brake W. G.. 2014. “Medial Prefrontal Cortical Estradiol Rapidly Alters Memory System Bias in Female Rats: Ultrastructural Analysis Reveals Membrane‐Associated Estrogen Receptors as Potential Mediators.” Endocrinology 155, no. 11: 4422–4432. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

