Intraspecific Sensory Diversity and the Decapod Claw: Patterns of Sensillation Are Heterochelic and Sexually Dimorphic In Pagurus bernhardus
- PMID: 40360456
- PMCID: PMC12075039
- DOI: 10.1002/jmor.70054
Intraspecific Sensory Diversity and the Decapod Claw: Patterns of Sensillation Are Heterochelic and Sexually Dimorphic In Pagurus bernhardus
Abstract
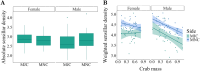
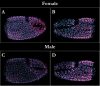
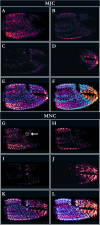
Information detection affects physiological performance and behaviour and is vital to survival and fitness. Despite the recognised importance of sensory adaptations in information acquisition and manipulation, many forms of sensory variation-from within individuals to between species-remain underexplored. To better understand the role of information in evolution, it is important to examine sensory variation as part of a cohesive framework of sensory diversity. Using the decapod claw, a structure well-recognised for its morphological variation, we investigated sensory diversity at the intraspecific level by assessing heterochely and sexual dimorphism in the chelar morphologies of Pagurus bernhardus hermit crabs. We employed a novel methodology using scanning electron microscopy (SEM) to assess moulted chelar tissue from both the major and minor claws. The shape, size, and sensillation (i.e., the distribution and abundance of sensilla) of both chelipeds were examined by geometric morphometric landmark analysis (GMLA), generalised Procrustes analysis (GPA), and linear mixed effects models. Hermit crabs exhibited heterochely and sexual dimorphism in both gross and sensory chelar morphologies. Sexual dimorphism was greater in the sensory morphology of the major claw, suggesting sex-based sensory specialisations, likely due to differences in mating roles and behaviours. In contrast, the minor claw's sensory morphology lacked sexual dimorphism, suggesting the sensory role of this appendage is equally important for both sexes. Our results highlight sensory variation as a fundamental aspect of functional morphology and emphasise the need to consider sexual dimorphism and body asymmetry in information acquisition. These findings contribute to a broader framework for studying sensory diversity, underscoring the importance of integrating sensory morphology, function, and ecology to fully understand the evolutionary implications of sensory specialisations.
Keywords: chela; crustacea; hermit crab; scanning electron microscopy (SEM); sensilla; sensory morphology; sexual dimorphism.
© 2025 The Author(s). Journal of Morphology published by Wiley Periodicals LLC.
Figures



References
-
- Akhter, E. T. , Pereira A., Hughes M., and Korey C. A.. 2015. “Plasticity of External Setae During Claw Transformation in the Snapping Shrimp, Alpheus angulosus McClure, 2002 (Decapoda, Caridea).” Crustaceana 88, no. 7–8: 893–910. 10.1163/15685403-00003457. - DOI
-
- Alencar, C. E. R. D. , Lima‐Filho P. A., Molina W. F., and Freire F. a M.. 2014. “Sexual Shape Dimorphism of the Mangrove Crab Ucides cordatus (Linnaeus, 1763) (Decapoda, Ucididae) accessed Through Geometric Morphometric.” Scientific World Journal 2014, no. 1: 206168. 10.1155/2014/206168. - DOI - PMC - PubMed
-
- ASAB Ethical Committee/ABS Animal Care Committee . 2023. “Guidelines for the Ethical Treatment of Nonhuman Animals in Behavioural Research and Teaching.” Animal Behaviour 195: I–XI. 10.1016/j.anbehav.2022.09.006. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

