GDF8 and activin A are the key negative regulators of muscle mass in postmenopausal females: a randomized phase I trial
- PMID: 40360471
- PMCID: PMC12075688
- DOI: 10.1038/s41467-025-59380-3
GDF8 and activin A are the key negative regulators of muscle mass in postmenopausal females: a randomized phase I trial
Abstract
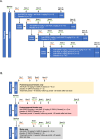
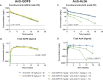
Evolutionary pressures to protect against food scarcity likely resulted in highly-conserved pathways designed to minimize energy expenditure, one of which involves the minimization of muscle mass; these mechanisms may be counter-productive in a modern world suffering from obesity and sarcopenia. Growth differentiation factor 8 (GDF8)/myostatin, acting via ActRIIA/B receptors, is the best-characterized negative regulator of muscle mass, leading to therapeutic efforts to augment muscle growth by blocking GDF8 or ActRIIA/B. ActRIIA/B blockade approximately doubles the muscle increase of GDF8 blockade, and as ActRIIA/B responds to multiple other TGFβ-family members, this implies other ligands might also regulate muscle mass. Previously, we suggested that activin A (ActA) is the key second negative regulator acting via ActRIIA/B, as blockade of both GDF8 and ActA in mice/monkeys matches the muscle growth of ActRIIA/B blockade. Here, we extend these observations to humans in a two-part, randomized, placebo-controlled Phase 1 trial ( www.clinicaltrials.gov , NCT02943239) conducted at two sites in New Zealand. Eligible subjects included healthy postmenopausal females aged 45-70 years and males aged 35-60 years not intending to father children, with a body mass index of 18-32 kg/m2. Part I tested single-dose administration of anti-GDF8 alone, anti-ActA alone, several dose combinations of anti-GDF8 + anti-ActA, or placebo in healthy postmenopausal females; part II tested multiple-dose administration of anti-ActA alone or placebo in healthy postmenopausal females, combination anti-GDF8 + anti-ActA or placebo in healthy postmenopausal females, and anti-ActA alone or placebo in healthy males. The primary outcome measure was the incidence and severity of treatment-emergent adverse events through week 16 for the single-dose part of the study and through week 40 for the multiple-dose part of the study. Secondary endpoints included percent and absolute change in thigh muscle volume, percent and absolute change in total and regional body composition, pharmacokinetic profiles of the GDF8 and ActA mAbs in serum over time, changes in serum total GDF8 and total ActA levels over time, and the presence of anti-drug antibodies against the GDF8 mAb or the ActA mAb. Magnetic resonance imaging was used to quantitate changes in thigh muscle volume and dual x-ray absorptiometry was used to quantitate changes in regional body composition (total lean mass, appendicular lean body mass, android fat mass, and total fat mass). A total of 82 subjects were enrolled (48 in the single-dose part and 34 in the multiple-dose part of the study). Baseline demographic and clinical characteristics were generally balanced across the single- and multiple-dose parts of the study. Combining GDF8 and ActA blocking antibodies led to greater muscle growth than either antibody alone; increases in muscle were accompanied by reductions in fat. The observed clinical effects on muscle and fat paralleled mAb exposure in serum. The combination was generally well tolerated, and no subjects tested positive for anti-drug antibodies post-treatment. These results suggest that GDF8 and ActA are the dominant negative regulators of muscle mass in humans, and that combined blockade may be a promising therapeutic approach in muscle atrophy and obesity settings.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.G.T., S.A., P.P., A.B., K.M., B.J.M., P.M., D.J.G., E.G., J.T., J.D.D., B.H., R.P., G.D.Y., and G.A.H. are employees and stockholders of Regeneron Pharmaceuticals, Inc. J.M. and M.W.S. are employees and stockholders of Regeneron Pharmaceuticals, Inc. who report having a patent pending, which has been licensed and receiving royalties with Regeneron Pharmaceuticals, Inc. S.D. was an employee of and holds stocks in Regeneron Pharmaceuticals, Inc. C.W. is an employee of and shareholder in New Zealand Clinical Research. The authors declare no other competing interests.
Figures


References
-
- Weyer, C., Snitker, S., Rising, R., Bogardus, C. & Ravussin, E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. Int. J. Obes. Relat. Metab. Disord.23, 715–722 (1999). - PubMed
-
- McCrory, M. A., Harbaugh, A. G., Appeadu, S. & Roberts, S. B. Fast-food offerings in the United States in 1986, 1991, and 2016 show large increases in food variety, portion size, dietary energy, and selected micronutrients. J. Acad. Nutr. Diet.119, 923–933 (2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

