Exosomal transfer of pro-pyroptotic miR-216a-5p exacerbates anthracycline cardiotoxicity through breast cancer-heart pathological crosstalk
- PMID: 40360476
- PMCID: PMC12075849
- DOI: 10.1038/s41392-025-02245-4
Exosomal transfer of pro-pyroptotic miR-216a-5p exacerbates anthracycline cardiotoxicity through breast cancer-heart pathological crosstalk
Abstract
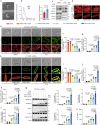
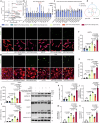
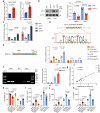
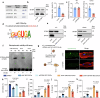
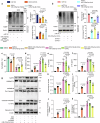
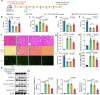
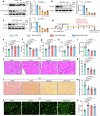
Doxorubicin (DOX) is the most effective chemotherapeutic for breast cancer, but it is usually associated with severe cardiotoxicity. Further investigation to alleviate its side effects is essential. The present study investigated the mechanism of the cross-organ communication between tumors and the heart and potential intervention targets. Morphological bubble-like protrusions were observed in both adult murine ventricular cardiomyocytes (AMVCs) and human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) cocultured with breast cancer cells (BCCs), along with elevated expression of pyroptosis-related proteins. Exosomes (EXOs) from DOX-treated BCCs aggravated DOX-induced cardiotoxicity (DOXIC) in an orthotopic mouse model of breast cancer. Blocking miRNAs by knocking down Rab27a or inhibiting the release of EXOs in cancer tissue by Dicer enzyme knockout attenuated this additional injury effect. Exosomal miRNA sequencing revealed that miR-216a-5p is especially upregulated in EXOs from DOX-induced BCCs. Mechanistically, miR-216a-5p was upregulated by enhanced transcription mediated by DOX-induced AMP-dependent transcription factor 3 (ATF3) and packaged into EXOs by splicing factor 3b subunit 4 (SF3B4) in BCCs. Itchy E3 ubiquitin-protein ligase (ITCH) was identified as a novel downstream target mRNA of miR-216a-5p. ITCH negatively mediated thioredoxin-interacting protein (TXNIP) ubiquitination to activate the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome pathway, ultimately leading to cardiomyocyte pyroptosis. Our findings revealed novel cross-organ pathogenic communication between breast cancer and the heart through the exosomal miR-216a-5p-mediated ITCH/TXNIP/NLRP3 pathway, which drives cardiomyocyte pyroptosis. These findings suggest that targeting myocardial miR-216a-5p or blocking harmful EXOs from breast cancer is a potential therapeutic strategy for alleviating DOXIC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Exosomal miR-202-5p derived from iPSC-MSCs protects against myocardial infarction through inhibition of cardiomyocyte pyroptosis.Stem Cell Res Ther. 2025 Jun 3;16(1):282. doi: 10.1186/s13287-025-04390-7. Stem Cell Res Ther. 2025. PMID: 40462173 Free PMC article.
-
The Tumor-Suppressive Human Circular RNA CircITCH Sponges miR-330-5p to Ameliorate Doxorubicin-Induced Cardiotoxicity Through Upregulating SIRT6, Survivin, and SERCA2a.Circ Res. 2020 Jul 31;127(4):e108-e125. doi: 10.1161/CIRCRESAHA.119.316061. Epub 2020 May 11. Circ Res. 2020. PMID: 32392088
-
Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis.Am J Physiol Heart Circ Physiol. 2019 Aug 1;317(2):H460-H471. doi: 10.1152/ajpheart.00056.2019. Epub 2019 Jun 7. Am J Physiol Heart Circ Physiol. 2019. PMID: 31172809 Free PMC article.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Use of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Preclinical Cancer Drug Cardiotoxicity Testing: A Scientific Statement From the American Heart Association.Circ Res. 2019 Oct 25;125(10):e75-e92. doi: 10.1161/RES.0000000000000291. Epub 2019 Sep 19. Circ Res. 2019. PMID: 31533542 Free PMC article. Review.
Cited by
-
Doxorubicin-Induced Cardiotoxicity: A Comprehensive Update.J Cardiovasc Dev Dis. 2025 May 30;12(6):207. doi: 10.3390/jcdd12060207. J Cardiovasc Dev Dis. 2025. PMID: 40558642 Free PMC article. Review.
References
-
- López-Fernández, T. et al. Breast cancer and cardiovascular health. Eur. Heart J.45, 4366–4382 (2024). - PubMed
MeSH terms
Substances
Grants and funding
- 2022YFA1104500/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 2022YFC3602400/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 82100372/National Natural Science Foundation of China (National Science Foundation of China)
- 82470366/National Natural Science Foundation of China (National Science Foundation of China)
- 7232159/Beijing Municipal Science and Technology Commission
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

