Psychological stress-induced systemic corticosterone directly sabotages intestinal stem cells and exacerbates colitis
- PMID: 40360481
- PMCID: PMC12075755
- DOI: 10.1038/s41421-025-00796-y
Psychological stress-induced systemic corticosterone directly sabotages intestinal stem cells and exacerbates colitis
Abstract
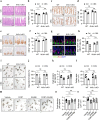
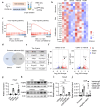
Psychological stress has profound impacts on the gastrointestinal tract via the brain‒gut axis. However, its effects on intestinal stem cells (ISCs) and the resulting implication for intestinal homeostasis remain poorly understood. Here, we observed a notable reduction in both the quantity and proliferative capacity of ISCs under chronic stress conditions, driven by elevated levels of corticosterone resulting from activation of the hypothalamic‒pituitary‒adrenal (HPA) axis. Mechanistically, corticosterone directly interacts with its receptor, nuclear receptor subfamily 3 group c member 1 (NR3C1), leading to increased expression of FKBP prolyl isomerase 5 (FKBP5) in ISCs. Subsequently, FKBP5 negatively regulates AKT activation by facilitating its dephosphorylation at Ser473, ultimately enhancing nuclear translocation of forkhead box O (FoxO) and inhibiting ISC proliferative activity. Consequently, ISC dysfunction contributes to the stress-driven exacerbation of DSS-induced colitis. Collectively, these findings reveal an intrinsic brain-to-gut regulatory pathway whereby psychological stress impairs ISC activity via corticosterone elevation, providing a mechanistic explanation for stress-enhanced susceptibility to colitis.
© 2025. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

