SARS-CoV-2 virus lacking the envelope and membrane open-reading frames as a vaccine platform
- PMID: 40360482
- PMCID: PMC12075476
- DOI: 10.1038/s41467-025-59533-4
SARS-CoV-2 virus lacking the envelope and membrane open-reading frames as a vaccine platform
Abstract
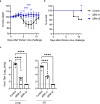
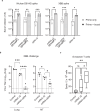
To address the need for broadly protective SARS-CoV-2 vaccines, we developed an attenuated a SARS-CoV-2 vaccine virus that lacks the open reading frames of two viral structural proteins: the envelope (E) and membrane (M) proteins. This vaccine virus (ΔEM) replicates in a cell line stably expressing E and M but not in wild-type cells. Vaccination with ΔEM elicits a CD8 T-cell response against the viral spike and nucleocapsid proteins. Two vaccinations with ΔEM provide better protection of the lower respiratory tissues than a single dose against the Delta and Omicron XBB variants in hamsters. Moreover, ΔEM is effective as a booster in hamsters previously vaccinated with an mRNA-based vaccine, providing higher levels of protection in both respiratory tissues compared to the mRNA vaccine booster. Collectively, our data demonstrate the feasibility of a SARS-CoV-2 ΔEM vaccine candidate virus as a vaccine platform.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Y.K. has received unrelated funding support from Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc., Shionogi & Co. LTD, Otsuka Pharmaceutical, KM Biologics, Kyoritsu Seiyaku, Shinya Corporation, and Fuji Rebio. The other authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- P01AI165077/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- JP23wm0125002/Japan Agency for Medical Research and Development (AMED)
- JP233fa627001/Japan Agency for Medical Research and Development (AMED)
- JP233fa827005/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

