Desymmetric esterification catalysed by bifunctional chiral N-heterocyclic carbenes provides access to inherently chiral calix[4]arenes
- PMID: 40360493
- PMCID: PMC12075856
- DOI: 10.1038/s41467-025-59781-4
Desymmetric esterification catalysed by bifunctional chiral N-heterocyclic carbenes provides access to inherently chiral calix[4]arenes
Abstract
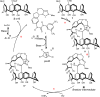
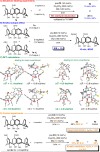
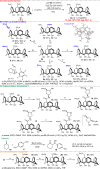
Calix[4]arenes display inherent chirality, with broad applications in synthetic and medicinal chemistry and in materials sciences. However, their use is hindered by their limited synthetic accessibility, primarily due to the lack of enantioselective methods for preparing chiral calix[4]arenes with an ABCC substitution pattern. Here, we address this challenge by presenting a simple, efficient, and metal-free protocol for organocatalytic desymmetrisation of prochiral diformylcalix[4]arenes. Through this highly effective and sustainable approach, we synthesize structurally unique products in gram-scale reactions. Accordingly, this method facilitates extensive post-functionalisations of the carbonyl groups, including for organocatalyst development. Furthermore, our experimental mechanistic studies demonstrate that desymmetrisation determines enantiocontrol in esterification reactions catalysed by N-heterocyclic carbenes. These findings underscore the broad potential of this method for providing versatile access to inherently chiral calix[4]arenes with an ABCC substitution pattern while offering a valuable platform for asymmetric molecular recognition and catalysis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Catalytic Enantioselective Synthesis of Inherently Chiral Calix[4]arenes via Sequential Povarov Reaction and Aromatizations.Angew Chem Int Ed Engl. 2024 Nov 4;63(45):e202410628. doi: 10.1002/anie.202410628. Epub 2024 Sep 2. Angew Chem Int Ed Engl. 2024. PMID: 38973580
-
Catalytic enantioselective synthesis of inherently chiral calix[4]arenes via organocatalyzed aromatic amination enabled desymmetrization.Nat Commun. 2025 Apr 26;16(1):3943. doi: 10.1038/s41467-025-59221-3. Nat Commun. 2025. PMID: 40287403 Free PMC article.
-
Organocatalytic Enantioselective Synthesis of Inherently Chiral Calix[4]arenes.Angew Chem Int Ed Engl. 2024 Sep 23;63(39):e202407752. doi: 10.1002/anie.202407752. Epub 2024 Jul 17. Angew Chem Int Ed Engl. 2024. PMID: 38844430
-
Enantioselective Catalytic Synthesis of Inherently Chiral Calixarenes.Chem Rec. 2025 Apr;25(4):e202400237. doi: 10.1002/tcr.202400237. Epub 2025 Jan 28. Chem Rec. 2025. PMID: 39876669 Review.
-
Inherently Chiral Calix[4]Arene Derivatives: Concept and Enantioselective Synthesis.Chemistry. 2025 Aug 25:e02247. doi: 10.1002/chem.202502247. Online ahead of print. Chemistry. 2025. PMID: 40855627 Review.
Cited by
-
Enantioselective lactonization catalyzed by chiral N-heterocyclic carbenes enables access to inherently chiral eight-membered lactones.Chem Sci. 2025 Aug 25. doi: 10.1039/d5sc05037e. Online ahead of print. Chem Sci. 2025. PMID: 40901621 Free PMC article.
References
-
- Hopkinson, M. M., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature510, 485–496 (2014). - PubMed
-
- Liu, Y., Wang, Y., Wu, X. & Chi, Y. R. Exploring molecular complexity by N-heterocyclic carbene organocatalysis: new activation and reaction diversity. Chem. Rec.23, e202200219 (2023). - PubMed
-
- Chen, X., Wang, H., Jin, Z. & Chi, Y. R. N-heterocyclic carbene organocatalysis: activation modes and typical reactive intermediates. Chin. J. Chem.38, 1167–1202 (2020).
-
- Shee, S., Ghosh, A. & Biju, A. T. Asymmetric Organocatalysis: New Strategies, Catalysts, and Opportunities, Vol. 10, 768 (Wiley, 2022).
Grants and funding
LinkOut - more resources
Full Text Sources

