FABP4 inhibition suppresses bone resorption and protects against postmenopausal osteoporosis in ovariectomized mice
- PMID: 40360512
- PMCID: PMC12075751
- DOI: 10.1038/s41467-025-59719-w
FABP4 inhibition suppresses bone resorption and protects against postmenopausal osteoporosis in ovariectomized mice
Abstract
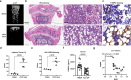
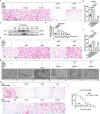
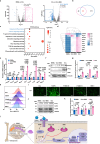
Postmenopausal osteoporosis (PMOP) is a condition in women caused by estrogen deficiency, characterized by reduced bone mass and increased fracture risk. Fatty acid-binding protein 4 (FABP4), a lipid-binding protein involved in metabolism and inflammation, has emerged as a key regulator in metabolic disorders and bone resorption; however, its direct role in PMOP remains unclear. Here, we show that serum FABP4 levels in PMOP patients negatively correlate with bone mineral density, a trend also observed in ovariectomized mice. FABP4 promotes osteoclast formation and bone resorption without affecting osteoblast differentiation. The FABP4 inhibitor BMS309403 suppresses osteoclast differentiation by modulating calcium signaling and inhibiting the Ca2+-Calcineurin-NFATc1 pathway. Oral BMS309403 increases bone mineral density in ovariectomized mice, though less effectively than alendronate. Notably, bone-targeted delivery of BMS309403 achieves comparable efficacy to alendronate. In this work, we demonstrate that FABP4 is a critical mediator in PMOP and that its inhibition offers a promising therapeutic strategy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Neer, R. M. et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med.344, 1434–1441 (2001). - PubMed
-
- Ebeling, P. R. et al. Secondary Osteoporosis. Endocr. Rev.43, 240–313 (2022). - PubMed
-
- Watts, N. B. Postmenopausal Osteoporosis: A Clinical Review. J. Women’s Health27, 1093–1096 (2018). - PubMed
-
- Wade, S. W., Strader, C., Fitzpatrick, L. A., Anthony, M. S. & O’Malley, C. D. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch. Osteoporos.9, 182 (2014). - PubMed
-
- Reginster, J. Y. & Burlet, N. Osteoporosis: A still increasing prevalence. Bone38, 4–9 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

