High-contrast in vivo fluorescence imaging exploiting wavelengths beyond 1880 nm
- PMID: 40360524
- PMCID: PMC12075662
- DOI: 10.1038/s41467-025-59630-4
High-contrast in vivo fluorescence imaging exploiting wavelengths beyond 1880 nm
Abstract
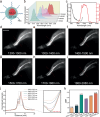
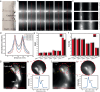
The second near-infrared (NIR-II) window is widely acknowledged for its excellent potential in in vivo fluorescence imaging. Currently, NIR-II fluorescence imaging predominantly operates within the 900-1880 nm spectral range, while the region beyond 1880 nm has been disregarded due to the large light absorption of water. Based on a refined understanding of the effect of light absorption on imaging, we propose an approach that utilizes the previously neglected region surrounding the water absorption peak at ~1930 nm for imaging. Both simulations and experiments confirm that the water absorption contributes positively to imaging, enabling high-contrast in vivo fluorescence imaging in the 1880-2080 nm window. To further assess the applicability of this approach in different biological media, we extend our focus to fluorescence imaging in adipose tissue. This leads to the expansion of the imaging window to 1700-2080 nm, owing to the unique light absorption characteristics of adipose tissue. Our results demonstrate that the 1700-2080 nm region provides optimal imaging quality in adipose tissue, attributing to its moderate absorption and low scattering. This work advances our understanding of the interplay between light absorption and photon scattering in bioimaging, providing an insight for selecting optimal imaging windows to achieve high-contrast fluorescence imaging.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Hu, Z. et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng.4, 259–271 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

