Chaperone-mediated autophagy manipulates PGC1α stability and governs energy metabolism under thermal stress
- PMID: 40360527
- PMCID: PMC12075589
- DOI: 10.1038/s41467-025-59618-0
Chaperone-mediated autophagy manipulates PGC1α stability and governs energy metabolism under thermal stress
Abstract
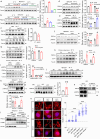
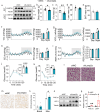
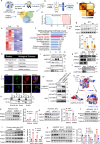
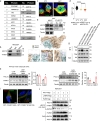
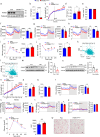
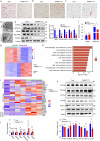
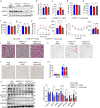
Thermogenic proteins are down-regulated under thermal stress, including PGC1α· However, the molecular mechanisms are not fully understood. Here, we addressed that chaperone-mediated autophagy could regulate the stability of PGC1α under thermal stress. In mice, knockdown of Lamp2a, one of the two components of CMA, in BAT showed increased PGC1α protein and improved metabolic phenotypes. Combining the proteomics of brown adipose tissue (BAT), structure prediction, co-immunoprecipitation- mass spectrum and biochemical assays, we found that PARK7, a Parkinson's disease causative protein, could sense the temperature changes and interact with LAMP2A and HSC70, respectively, subsequently manipulate the activity of CMA. Knockout of Park7 specific in BAT promoted BAT whitening, leading to impaired insulin sensitivity and energy expenditure at thermoneutrality. Moreover, inhibiting the activity of CMA by knockdown of LAMP2A reversed the effects induced by Park7 ablation. These findings suggest CMA is required for BAT to sustain thermoneutrality-induced whitening through degradation of PGC1α.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

