Effects of ulinastatin on therapeutic outcomes and inflammatory markers in pediatric septic shock patients
- PMID: 40360546
- PMCID: PMC12075584
- DOI: 10.1038/s41598-025-00629-8
Effects of ulinastatin on therapeutic outcomes and inflammatory markers in pediatric septic shock patients
Abstract
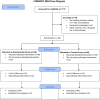
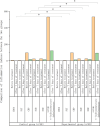
This study aims to investigate the effects of ulinastatin on therapeutic outcomes and inflammatory markers in children with septic shock. This study selected 117 children with septic shock admitted to our hospital from April 2023 to April 2024. Among them, 7 children had comorbidities, 3 dropped out during the research process, 5 did not continue to participate in the study due to other reasons, and 4 explicitly refused to participate. After screening and excluding the above - mentioned cases, A total of 98 children were allotted to experimental group (ulinastatin combined with routine antishock therapy, n = 49) and control group (routine antishock therapy, n = 49) using stratified random method. The therapeutic effects and levels of inflammatory markers were assessed before and after treatment using a double antibody sandwich ELISA. The outcome was significantly better in the experimental group compared to controls (P < 0.05). Prior to treatment, there were no significant differences in the levels of CD64, procalcitonin (PCT), C-reactive protein, neutrophil-to-lymphocyte ratio (NLR), and serum α-hydroxybutyrate dehydrogenase between the two groups (all P > 0.05). After 7 days of treatment, the experimental group exhibited significantly lower levels of CD64, PCT, C-reactive protein, NLR, and serum α-hydroxybutyrate dehydrogenase compared to the control group (all P < 0.001). Ulinastatin enhances the therapeutic efficacy in children with septic shock by reducing levels of associated inflammatory markers and promoting recovery.
Keywords: Inflammatory indicators; Pediatric patients; Septic shock; Therapeutic effect; Ulinastatin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The study protocol was approved by the Ethics Committee of Huanggang Central Hospital of Yangtze University, and the study was performed in accordance with the Helsinki II declaration. Informed consent was obtained from all the study subjects before enrollment.
Figures
Similar articles
-
Clinical efficacy of ulinastatin in the treatment of unliquefied pyogenic liver abscess complicated by septic shock: A randomized controlled trial.Immun Inflamm Dis. 2023 Apr;11(4):e822. doi: 10.1002/iid3.822. Immun Inflamm Dis. 2023. PMID: 37102655 Free PMC article. Clinical Trial.
-
Effectiveness and tolerability of pharmacologic and combined interventions for reducing injection pain during routine childhood immunizations: systematic review and meta-analyses.Clin Ther. 2009;31 Suppl 2:S104-51. doi: 10.1016/j.clinthera.2009.08.001. Clin Ther. 2009. PMID: 19781433
-
Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections.Cochrane Database Syst Rev. 2017 Oct 12;10(10):CD007498. doi: 10.1002/14651858.CD007498.pub3. Cochrane Database Syst Rev. 2017. PMID: 29025194 Free PMC article.
-
Biomarkers as point-of-care tests to guide prescription of antibiotics in people with acute respiratory infections in primary care.Cochrane Database Syst Rev. 2022 Oct 17;10(10):CD010130. doi: 10.1002/14651858.CD010130.pub3. Cochrane Database Syst Rev. 2022. PMID: 36250577 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Wang, N. et al. The role of eSOFA score in 28-day, 90-day and 1-year prognosis assessment of patients with sepsis. Chin. Emerg. Med.43(09), 711–715 (2023).
-
- Patel, J. J., Shukla, A. & Heyland, D. K. Enteral nutrition in septic shock: A pathophysiologic conundrum. JPEN J. Parenter. Enter. Nutr.45(S2), 74–78 (2021). - PubMed
-
- De Backer, D., Ricottilli, F. & Ospina-Tascón, G. A. Septicshock: Amicro circulation disease. Curr. Opin. Anaesthesiol.34(2), 85–91 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous