Bufei Yishen formula alleviates airway epithelial cell senescence in COPD by activating AMPK-Sirt1-FoxO3a pathway and promoting autophagy
- PMID: 40360552
- PMCID: PMC12075830
- DOI: 10.1038/s41598-025-00746-4
Bufei Yishen formula alleviates airway epithelial cell senescence in COPD by activating AMPK-Sirt1-FoxO3a pathway and promoting autophagy
Abstract
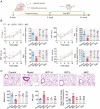
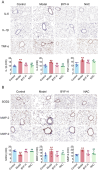
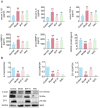
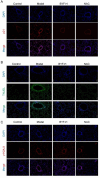
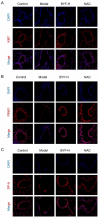
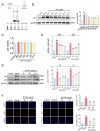
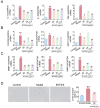
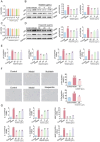
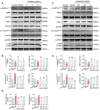
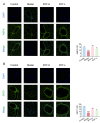
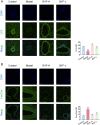
The Bufei Yishen formula (BYF) has traditionally been employed to treat patients with COPD, demonstrating significant effectiveness. However, the underlying mechanisms through which BYF alleviates COPD remains unclear. Cellular senescence is crucial in the pathogenesis of COPD. This study aims to investigate whether the therapeutic mechanism of BYF is associated with the reduction of cellular senescence. To evaluate the anti-senescence effects of BYF, a COPD rat model and a cellular senescence model were established. The active compounds and underlying mechanisms of BYF were investigated in vitro. BYF treatment significantly mitigated lung function decline and pathological damage in COPD rats. It significantly inhibited senescence in lung tissue by decreasing the expression of the cell cycle inhibitor p21, DNA damage markers, pro-inflammatory cytokines, and matrix metalloproteinases. BYF4/5, isolated from BYF, demonstrated significant anti-senescence effects in bronchial epithelial cells. Additionally, 67 compounds were identified from BYF4/5, and 770 targets were predicted for these compounds. hesperidin and nobiletin, identified as key compounds in BYF, were found to inhibit cellular senescence and activate the AMPK-Sirt1-FoxO3a pathway and autophagy in 16HBE cells. The data indicate that BYF alleviates COPD by activating the AMPK-Sirt1-FoxO3a pathway and autophagy, thereby inhibiting bronchial epithelial cell senescence.
Keywords: AMPK; Autophagy; Bufei Yishen formula; Cellular senescence; Chronic obstructive pulmonary disease; FoxO3a; Sirt1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Zuo, L. & Wijegunawardana, D. Redox Role of ROS and Inflammation in Pulmonary Diseases. In Lung Inflammation in Health and Disease, Volume II (ed. Wang, Y. X.) 187–204 (Springer International Publishing, 2021). 10.1007/978-3-030-68748-9_11. - PubMed
MeSH terms
Substances
Grants and funding
- No. 82274274, 81904165, 81973822/National Natural Science Fund of China
- No. 82274274, 81904165, 81973822/National Natural Science Fund of China
- No. 82274274, 81904165, 81973822/National Natural Science Fund of China
- No. 82274274, 81904165, 81973822/National Natural Science Fund of China
- No. 82274274, 81904165, 81973822/National Natural Science Fund of China
- No. 82274274, 81904165, 81973822/National Natural Science Fund of China
- No. 222301420020/Joint Fund for Science and Technology Research of Henan Province
- No. 222301420020/Joint Fund for Science and Technology Research of Henan Province
- No. 222301420020/Joint Fund for Science and Technology Research of Henan Province
- No. 222301420020/Joint Fund for Science and Technology Research of Henan Province
- No. 222301420020/Joint Fund for Science and Technology Research of Henan Province
- No. 222301420020/Joint Fund for Science and Technology Research of Henan Province
- No.22301420070/joint Fund for Science and Technology Research of Henan Province
- No.22301420070/joint Fund for Science and Technology Research of Henan Province
- No.22301420070/joint Fund for Science and Technology Research of Henan Province
- No.22301420070/joint Fund for Science and Technology Research of Henan Province
- No.22301420070/joint Fund for Science and Technology Research of Henan Province
- No.22301420070/joint Fund for Science and Technology Research of Henan Province
- No. 2023GGJS084/Training Program for Young Teachers in Henan's University
- No. 2023GGJS084/Training Program for Young Teachers in Henan's University
- No. 2023GGJS084/Training Program for Young Teachers in Henan's University
- No. 2023GGJS084/Training Program for Young Teachers in Henan's University
- No. 2023GGJS084/Training Program for Young Teachers in Henan's University
- No. 2023GGJS084/Training Program for Young Teachers in Henan's University
- No. 24HASTIT073/Henan Education Science and Technology Innovation Talent Support Program
- No. 24HASTIT073/Henan Education Science and Technology Innovation Talent Support Program
- No. 24HASTIT073/Henan Education Science and Technology Innovation Talent Support Program
- No. 24HASTIT073/Henan Education Science and Technology Innovation Talent Support Program
- No. 24HASTIT073/Henan Education Science and Technology Innovation Talent Support Program
- No. 24HASTIT073/Henan Education Science and Technology Innovation Talent Support Program
LinkOut - more resources
Full Text Sources
Medical
Research Materials

