Development and validation of a cancer-associated fibroblast gene signature-based model for predicting immunotherapy response in colon cancer
- PMID: 40360558
- PMCID: PMC12075585
- DOI: 10.1038/s41598-025-01185-x
Development and validation of a cancer-associated fibroblast gene signature-based model for predicting immunotherapy response in colon cancer
Abstract
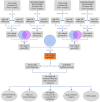
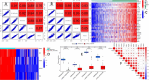
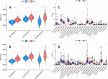
The efficacy of immune checkpoint inhibitors in colon cancer has been established, and there is an urgent need to identify new molecular markers for colon cancer immunotherapy to guide clinical decisions. Using the "EPIC" and "MCPcounter" R packages to conduct cancer-associated fibroblast (CAF) infiltration scoring on colon cancer samples from the TCGA database and the GEO database, the WGCNA analysis was performed on the two databases' samples based on the CAF infiltration scores to screen for CAF-related genes. LASSO regression analysis was used to construct a risk model with these genes. Comprehensive bioinformatics analysis was conducted on the constructed model to evaluate the stability of its prediction of CAF infiltration abundance and the stability of its prediction of immunotherapy efficacy. The newly constructed risk model could well reflect the abundance of CAF infiltration in colon cancer, with a correlation coefficient of 0.91 in the training cohort TCGA-COAD and 0.88 in the validation cohort GSE39582. GSEA analysis revealed that CAF is closely related to functions associated with extracellular matrix remodeling. The constructed risk model can predict the efficacy of immunotherapy in colon cancer well, with the high-risk group showing significantly poorer immunotherapy response than the low-risk group, with an expected effective rate of immunotherapy of 68 vs. 24% in the training group (P < 0.001) and 64 vs. 26% in the validation group (P < 0.001). The AUC value for predicting immunotherapy response by the risk model in the training group was 0.780 (95% CI 0.736-0.820), and in the validation group, the AUC value was 0.774 (95% CI 0.735-0.810). Drug sensitivity analysis showed that the expected chemotherapeutic effect in the low-risk group was superior to that in the high-risk group. CAF is associated with immunosuppression and drug resistance. Predicting the efficacy of immunotherapy in colon cancer based on the abundance of CAF infiltration is a feasible approach. For the high-risk population identified by our model, clinical consideration should be given to prioritizing non-immunotherapy approaches to avoid potential risks associated with immunotherapy.
Keywords: Cancer-associated fibroblasts (CAF); Colon cancer; Immunotherapy response prediction; Risk stratification model; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

