Pyrrole dicarboxylate substituted porphyrazine, microwave assisted synthesis and properties
- PMID: 40360570
- PMCID: PMC12075845
- DOI: 10.1038/s41598-025-00428-1
Pyrrole dicarboxylate substituted porphyrazine, microwave assisted synthesis and properties
Abstract
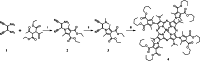
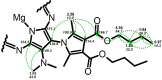
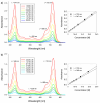
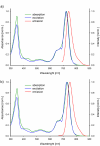
In this study, a new magnesium(II) porphyrazine derivative tetrasubstituted with dicarboxypyrrolyl moieties was synthesized. Two different approaches were used, utilizing conventional heating and microwave irradiation. The developed three-step processes were compared showing the superior performance of the microwave-assisted organic synthesis. The new macrocycle and the novel maleonitrile derivatives were characterized using spectral techniques (mass spectrometry, NMR spectroscopy, UV-Vis spectrophotometry) and the ability of the porphyrazine to generate singlet oxygen assessed using the method with 1,3-diphenylisobenzofuran. The singlet oxygen generation quantum yields were found to be moderate (ΦΔ = 0.23 and 0.22 in DMF and DMSO, respectively) and no aggregation behavior was noted in a series of dilutions. Additionally, the acute toxicity test using Microtox was performed showing almost no toxicity at the concentration of 10- 5 mol/L. The electrochemical studies revealed three redox processes of targeted porphyrazine with low first oxidation peak, whereas the spectroelectrochemistry showed the formation of both cationic and anionic species at proper potentials.
Keywords: Diaminomaleonitrile; Electrochemistry, aminoporphyrazines; Microwave-assisted organic synthesis; Porphyrazine; Singlet oxygen.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Synthesis, Electrochemical and Photochemical Properties of Sulfanyl Porphyrazine with Ferrocenyl Substituents.Molecules. 2023 Jul 5;28(13):5215. doi: 10.3390/molecules28135215. Molecules. 2023. PMID: 37446877 Free PMC article.
-
S-seco-porphyrazine as a new member of the seco-porphyrazine family - Synthesis, characterization and photocytotoxicity against cancer cells.Bioorg Chem. 2020 Mar;96:103634. doi: 10.1016/j.bioorg.2020.103634. Epub 2020 Jan 30. Bioorg Chem. 2020. PMID: 32044518
-
Nipagin-Functionalized Porphyrazine and Phthalocyanine-Synthesis, Physicochemical Characterization and Toxicity Study after Deposition on Titanium Dioxide Nanoparticles P25.Molecules. 2021 May 1;26(9):2657. doi: 10.3390/molecules26092657. Molecules. 2021. PMID: 34062815 Free PMC article.
-
Sulfanyl Porphyrazines with Morpholinylethyl Periphery-Synthesis, Electrochemistry, and Photocatalytic Studies after Deposition on Titanium(IV) Oxide P25 Nanoparticles.Molecules. 2021 Apr 15;26(8):2280. doi: 10.3390/molecules26082280. Molecules. 2021. PMID: 33920778 Free PMC article.
-
Tetra-2,3-pyrazinoporphyrazines with externally appended pyridine rings. 8. Central (Zn(II), Cu(II), Mg(II)(H2O), Cd(II)) and exocyclic (Pd(II)) metal ion binding in heteropentametallic complexes from tetrakis-2,3-[5,6-di(2-pyridyl)pyrazino]porphyrazine.Inorg Chem. 2010 Mar 1;49(5):2447-56. doi: 10.1021/ic902317h. Inorg Chem. 2010. PMID: 20102216
Cited by
-
Novel Tetraphenolic Porphyrazine Capable of MRSA Photoeradication.Molecules. 2025 Jul 22;30(15):3069. doi: 10.3390/molecules30153069. Molecules. 2025. PMID: 40807244 Free PMC article.
-
Phthalocyanines Conjugated with Small Biologically Active Compounds for the Advanced Photodynamic Therapy: A Review.Molecules. 2025 Aug 6;30(15):3297. doi: 10.3390/molecules30153297. Molecules. 2025. PMID: 40807472 Free PMC article. Review.
References
-
- Stuzhin, P. A. & Ercolani, C. Porphyrazines with annulated heterocycles. in The Porphyrin Handbook 263–364 (Elsevier, 10.1016/B978-0-08-092389-5.50011-0. (2003).
-
- Fuchter, M. J. et al. ROM Polymerization-Capture-Release strategy for the Chromatography-Free synthesis of novel unsymmetrical porphyrazines. J. Org. Chem.70, 2793–2802 (2005). - PubMed
-
- Mani, N. S. et al. Synthesis and characterisation of porphyrazinoctamine derivatives: X-ray crystallographic studies of [2,3,7,8,12,13,17,18-octakis(dibenzylamino)- porphyrazinato]magnesium(II) and {2,3,7,8,12,13,17,18-octakis[allyl(benzyl)amino]- porphyrazinato} nickel(II). J. Chem. Soc., Chem. Commun. (1994) (2095). 10.1039/c39940002095
-
- Fuchter, M. J. et al. Synthesis of porphyrazine-octaamine, hexamine and Diamine derivatives. Tetrahedron61, 6115–6130 (2005).
-
- Leng, F., Wang, X., Jin, L. & Yin, B. The synthesis and properties of unsymmetrical porphyrazines annulated with a tetrathiafulvalene bearing two tetraethylene glycol units. Dyes Pigm.87, 89–94 (2010).
Grants and funding
- JAK0000056/Uniwersytet Medyczny im. Karola Marcinkowskiego w Poznaniu
- JAK0000056/Uniwersytet Medyczny im. Karola Marcinkowskiego w Poznaniu
- JAK0000056/Uniwersytet Medyczny im. Karola Marcinkowskiego w Poznaniu
- JAK0000056/Uniwersytet Medyczny im. Karola Marcinkowskiego w Poznaniu
- JAK0000056/Uniwersytet Medyczny im. Karola Marcinkowskiego w Poznaniu
LinkOut - more resources
Full Text Sources

