Analysis of raltegravir analogs to enhance inhibitory efficiency against HIV integrase
- PMID: 40360594
- PMCID: PMC12075693
- DOI: 10.1038/s41598-025-01666-z
Analysis of raltegravir analogs to enhance inhibitory efficiency against HIV integrase
Abstract
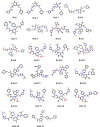
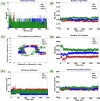
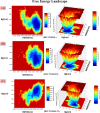
This article addresses the improvement of the efficacy of anti-integrase enzyme drugs for the AIDS virus, especially using the drug Raltegravir and its 21 analogs. In this research, Hartree-Fock and Density Functional Theory methods have been employed for the design and optimization of new drug candidates. These methods are used to enhance the accuracy and reactivity of the drugs. Additionally, docking is used to investigate the interactions between the drug and the target and evaluate binding energies. Molecular dynamics simulation is utilized to validate binding results. Computational results indicate that the designed analogs exhibit higher reactivity. In molecular docking calculations, RAL5 and RAL21 show the best binding energies of -10.10 and - 10.92 kcal/mol, respectively, indicating their superior efficiency. The analysis of inhibitor potentials against the HIV-1 integrase enzyme through molecular dynamics simulation reveals that RAL5 has strong inhibitory potential for treating viral diseases. These findings contribute to the promotion of therapeutic intervention methods in this field.
Keywords: Drug design; HIV-1 integrase; Raltegravir; Viral disease treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Investigation on the sucrose binding pocket of HIV-1 Integrase by molecular dynamics and synergy experiments.Bioorg Med Chem Lett. 2015 Aug 1;25(15):3013-6. doi: 10.1016/j.bmcl.2015.05.011. Epub 2015 May 15. Bioorg Med Chem Lett. 2015. PMID: 26048795
-
Molecular dynamics approaches estimate the binding energy of HIV-1 integrase inhibitors and correlate with in vitro activity.Antimicrob Agents Chemother. 2012 Jan;56(1):411-9. doi: 10.1128/AAC.05292-11. Epub 2011 Oct 28. Antimicrob Agents Chemother. 2012. PMID: 22037850 Free PMC article.
-
HIV integrase inhibitor, Elvitegravir, impairs RAG functions and inhibits V(D)J recombination.Cell Death Dis. 2017 Jun 1;8(6):e2852. doi: 10.1038/cddis.2017.237. Cell Death Dis. 2017. PMID: 28569776 Free PMC article.
-
Diketoacid inhibitors of HIV-1 integrase: from L-708,906 to raltegravir and beyond.Curr Med Chem. 2012;19(8):1177-92. doi: 10.2174/092986712799320565. Curr Med Chem. 2012. PMID: 22214459 Review.
-
Raltegravir, elvitegravir, and metoogravir: the birth of "me-too" HIV-1 integrase inhibitors.Retrovirology. 2009 Mar 5;6:25. doi: 10.1186/1742-4690-6-25. Retrovirology. 2009. PMID: 19265512 Free PMC article. Review.
References
-
- Kleinberg, M. L. & Wanke, L. A. New approaches and technologies in drug design and discovery. Am. J. Health Syst. Pharm.52 (12), 1323–1336 (1995). - PubMed
-
- Duarte, Y. et al. Integration of target discovery, drug discovery and drug delivery: a review on computational strategies. Wiley Interdisciplinary Reviews: Nanomed. Nanobiotechnol.11 (4), e1554 (2019). - PubMed
-
- Najafi, V., Yoosefian, M. & Hassani, Z. Development of venetoclax performance using its new derivatives on BCL-2 protein Inhibition. Cell Biochem. Funct.41 (1), 58–66 (2023). - PubMed
-
- Yoosefian, M., Moghani, M. Z. & Juan, A. In silico evaluation of atazanavir as a potential HIV main protease inhibitor and its comparison with new designed analogs. Comput. Biol. Med.145, 105523 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

