A pharmacovigilance analysis of post-marketing safety of durvalumab
- PMID: 40360595
- PMCID: PMC12075497
- DOI: 10.1038/s41598-025-01583-1
A pharmacovigilance analysis of post-marketing safety of durvalumab
Abstract
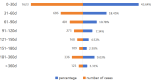
Durvalumab has demonstrated significant efficacy in several types of malignancies, while large-scale real-world safety studies remain limited. This study aimed to systematically evaluate the safety of durvalumab through data mining of the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). We extracted reports of durvalumab as the primary suspected drug from the FAERS database (January 2017 to June 2024). Four disproportionality analysis algorithms were used to detect signals between durvalumab and adverse events (AEs). Durvalumab was recorded in 10,120 reports as the primary suspected drug. Of these, 43.6% of AEs occurred during the first month of treatment, with a median onset time of 40 days (IQR: 14-99 ). Among 181 potential signals, 64 were unexpected preferred terms not listed in the prescribing information, including cytokine release syndrome (CRS), pulmonary tuberculosis, radiation esophagitis, oesophageal fistula, oesophageal perforation, pleural effusion, pneumothorax, cerebral infarction, biliary tract infection, cholecystitis, psoriasiform dermatitis, portal vein thrombosis, acute cholangitis and pericarditis malignant. Serious adverse events accounted for 93.3% of cases. Males exhibited a significantly higher risk of experiencing serious outcomes compared to females (OR = 1.83, 95% CI: 1.52-2.19, P < 0.001). Older age groups demonstrated an elevated risk of severe outcomes relative to those under 65 years (65-74 years: OR = 1.52, 95% CI: 1.15-2.00, P = 0.003; ≥75 years: OR = 1.40, 95% CI: 1.02-1.92, P = 0.038). This study comprehensively assessed the safety of durvalumab and discovered potential new adverse event signals, which may provide critical support for risk identification and monitoring of durvalumab.
Keywords: Adverse events; Cytokine-release syndrome; Durvalumab; FAERS; Pharmacovigilance; Pulmonary tuberculosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: Ethical approval was not required for this study because it used the FAERS database, which is a free open-access database.
Figures
References
-
- Kramer, R. et al. Hematological immune related adverse events after treatment with immune checkpoint inhibitors. Eur. J. Cancer. 147, 170–181. 10.1016/j.ejca.2021.01.013 (2021). - PubMed
-
- Zhao, Y. et al. Exploring the safety profile of Tremelimumab: an analysis of the FDA adverse event reporting system. Int. J. Clin. Pharm.46 (2), 480–487. 10.1007/s11096-023-01678-7 (2024). - PubMed
-
- Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol.16, 223–249. 10.1146/annurev-pathol-042020-042741 (2021). - PubMed
-
- Stewart, R. et al. Identification and characterization of MEDI4736, an antagonistic Anti-PD-L1 monoclonal antibody. Cancer Immunol. Res.3 (9), 1052–1062. 10.1158/2326-6066.CIR-14-0191 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical