Bacterial acidic agents-assisted multi-elemental (Ni, Co, and Li) leaching of used lithium-ion batteries at high pulp densities
- PMID: 40360650
- PMCID: PMC12075580
- DOI: 10.1038/s41598-025-00660-9
Bacterial acidic agents-assisted multi-elemental (Ni, Co, and Li) leaching of used lithium-ion batteries at high pulp densities
Abstract
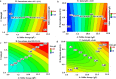
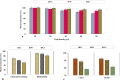
Accumulating used lithium-ion battery cathodes and associated environmental concerns necessitate efficient recycling strategies. Biohydrometallurgical processes often face challenges at high pulp densities due to microbial inhibition and substrate limitations, particularly sulfur availability, which is crucial for bacterial acidic agent production. This study introduces a breakthrough spent-medium bioleaching approach optimized for high-pulp-density conditions. We systematically addressed key challenges, including bacterial inhibition, sulfuric acid optimization, and its impact on critical metal dissolution. Using response surface methodology, we optimized sulfur dosage, inoculum size, and initial pH to enhance bacterial acidic agent production by Acidithiobacillus thiooxidans, achieving a sulfate concentration of 40.3 g/l and a ΔpH of 1.87. Metal removal efficiency was assessed at pulp densities of 10-50 g/l, demonstrating high extraction rates of Li (92%), Ni (88%), and Co (78%) at 50 g/l after 7 days. Comparative analysis with chemical leaching confirmed the effectiveness of this green strategy. Furthermore, a kinetic study using the Avrami equation and shrinking core model revealed that both models yield comparable results, and diffusion through the product layer controlled the leaching rate. This study presents a comprehensive and sustainable strategy for waste recycling at high pulp densities by integrating process optimization, spent-medium bioleaching, and kinetic modeling for critical metal extraction from lithium-ion battery cathodes.
Keywords: Acidithiobacillus thiooxidans; Bacterial acidic agent; Biohydrometallurgy; High pulp density; Response surface methodology; Used lithium-ion batteries.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Srichandan, H., Mohapatra, R. K., Parhi, P. K. & Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy189, 105122 (2019). - DOI
-
- Alipanah, M., Reed, D., Thompson, V., Fujita, Y. & Jin, H. Sustainable bioleaching of lithium-ion batteries for critical materials recovery. J. Clean. Prod.382, 135274 (2023). - DOI
-
- Roy, J. J., Madhavi, S. & Cao, B. Metal extraction from spent lithium-ion batteries (LIBs) at high pulp density by environmentally friendly bioleaching process. J. Clean. Prod.280, 124242 (2021). - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

