Edaravone dexborneol provides neuroprotective benefits by suppressing ferroptosis in experimental intracerebral hemorrhage
- PMID: 40360664
- PMCID: PMC12075699
- DOI: 10.1038/s41598-025-99187-2
Edaravone dexborneol provides neuroprotective benefits by suppressing ferroptosis in experimental intracerebral hemorrhage
Abstract
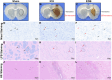
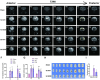
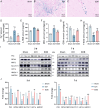
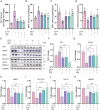
Edaravone dexborneol (EDB) is widely recognized for its anti-inflammatory and antioxidant properties and is clinically applied in the treatment of acute cerebral infarction. Ferroptosis is a critical process in the pathophysiology of brain injury following intracerebral hemorrhage (ICH). However, it remains unclear whether EDB can ameliorate ICH through the modulation of ferroptosis. This study aimed to evaluate the function and mechanism of EDB in treatment of ICH. With a male rat ICH model, animal behavior tests, histopathological staining, magnetic resonance imaging and evans blue staining were used to evaluate the neural protective function of EDB on ICH rats. The potential molecular mechanism was investigated using RNA sequencing. With the administration of Fer-1, a range of ferroptosis-related biomarkers, including Fe2+, 4-hydroxynonenal, malondialdehyde, etc., were analyzed to ascertain whether EDB confers neuroprotective effects through the modulation of P53/GPX4 pathways to inhibit ferroptosis. Finally, the findings were further corroborated using an in vitro ICH model with a P53 inhibitor. EDB has the potential to markedly enhance nerve and motor function, mitigate pathological damage, facilitate hematoma clearance, and repair BBB injury in ICH rats. KEGG analysis revealed that the differentially expressed genes were associated with signaling pathways, including P53 and ferroptosis. Both EDB and Fer-1 substantially reduced the concentrations of Fe2+, 4-hydroxynonenal, malondialdehyde, increased the amount of anti-oxidants, decreased the expression of P53, and concurrently upregulated the expression of GPX4. Besides, the P53 inhibitor PFT-α was observed to significantly reduce the levels of 4-HNE and lipid peroxides, while concurrently increasing the expression of GPX4. This investigation has shed light on the crucial neuroprotective role of EDB by regulating ferroptosis in ICH disease, which provided a theoretical basis for the clinical application of EDB in the treatment of ICH.
Keywords: Edaravone dexborneol; Ferroptosis; GPX4; Intracerebral hemorrhage; P53.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Dauricine alleviated secondary brain injury after intracerebral hemorrhage by upregulating GPX4 expression and inhibiting ferroptosis of nerve cells.Eur J Pharmacol. 2022 Jan 5;914:174461. doi: 10.1016/j.ejphar.2021.174461. Epub 2021 Aug 29. Eur J Pharmacol. 2022. PMID: 34469757
-
Edaravone dexborneol regulates γ-aminobutyric acid transaminase in rats with acute intracerebral hemorrhage.J Stroke Cerebrovasc Dis. 2024 Jul;33(7):107738. doi: 10.1016/j.jstrokecerebrovasdis.2024.107738. Epub 2024 May 1. J Stroke Cerebrovasc Dis. 2024. PMID: 38701940
-
AKR1C1 protects against intracerebral hemorrhage by suppressing neuronal cell death via the P53/SLC7A11/GPX4 axis.Brain Res Bull. 2025 Mar;222:111254. doi: 10.1016/j.brainresbull.2025.111254. Epub 2025 Feb 10. Brain Res Bull. 2025. PMID: 39938753
-
Potential of Edaravone Dexborneol in the treatment of cerebral ischemia: focus on cell death-related signaling pathways.Mol Biol Rep. 2024 Sep 23;51(1):1007. doi: 10.1007/s11033-024-09952-1. Mol Biol Rep. 2024. PMID: 39312062 Review.
-
Traditional Chinese Medicine and Ferroptosis in Intracerebral Hemorrhage: A Potential Therapeutic Approach.Drug Des Devel Ther. 2025 Jun 4;19:4789-4808. doi: 10.2147/DDDT.S513343. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40486126 Free PMC article. Review.
References
-
- Zhu, H. et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog. Neurobiol.178, 101610. 10.1016/j.pneurobio.2019.03.003 (2019). - PubMed
MeSH terms
Substances
Grants and funding
- XYBSKYZZ201902/Xinxiang Medical University Doctor Support Foundation
- 2024GGJS088/Henan Province Foundation for University Key Teacher
- 242300421199/Natural Science Foundation of Henan Province
- HNSJXF-2021-004/Henan Key Laboratory of Neurorestoratology
- 212102310835/Scientific and Technological Research Project of Henan Provincial Science and Technology Department
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

