Exploring corrosion behavior, antimicrobial evaluation, molecular docking and DFT calculation of thiosemicarbazone ligand and its metal complexes
- PMID: 40360666
- PMCID: PMC12075617
- DOI: 10.1038/s41598-025-98580-1
Exploring corrosion behavior, antimicrobial evaluation, molecular docking and DFT calculation of thiosemicarbazone ligand and its metal complexes
Abstract
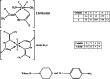
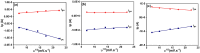
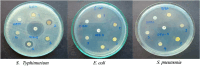
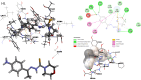
In the current study, the execution of thiosemicarbazone ligand (HL) as a novel corrosion inhibitor for copper metal in 1 M HCl solution was evaluated through the electrochemical measurements which includes (open circuit potential (OCP) potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS). The results confirmed that the ligand (HL) acted as a good corrosion inhibitor for copper metal in 1 M HCl solution; as it displayed high percentage of inhibition efficiency about 94.66% and 92.93% after PDP and EIS methods respectively; at its optimum concentration (1 × 10-7 M). The morphology and surface constituents of the sample were examined before and after addition of the ligand (HL) by using the analysis (scanning electron microscope and an energy dispersive X-ray spectroscopy) which clarified the passivation effect of the ligand (HL) after formation of a protective layer of its adsorbed molecules on the surface of the copper sample. In addition, the metal complexes Ni (II), Co (II) and Cd (II) derived from thiosemicarbazone ligand (HL) were used in this study to shed light on some of their electrochemical properties. But based on their nature as they are insoluble in aqueous media the cyclic voltammetry method was used in this section. The results deducted from cyclic voltammetry technique showed that, the oxidation-reduction process of the ligand (HL) and its metal complexes Ni (II), Co (II) and Cd (II) under quasi-reversible system and the reaction occurred on the metal surface under diffusion control. In vitro, the antibacterial activity testing against S. aureus, S. pneumonia, E. coli and S. Typhimurium were performed for the ligand (HL) and its metal complexes Ni (II), Co (II) and Cd (II). The result showed that Co (II) and Cd (II), complexes exhibited the best antibacterial activity against S. pneumonia, S. Typhimurium and E. coli while, all the compounds did not show any antibacterial activity against S. aureus. To obtain a good relation that supports and explains the interactions between the molecules of the studied compounds and the metal surface and with the antibacterial activity; the theoretical study in detail was applied using density functional theory (DFT) and molecular docking. The parameters such as, energy level (ΔE), the highest HOMO (EH), and the lowest occupied LUMO (EL), molecular orbital and the binding energy are deducted and discussed. The main target investigated of this study is that the thiosemicarbazone ligand (HL) can be used as a new corrosion inhibitor for the metals and their alloys against the aggressive media. Also, from cyclic voltammetry technique which had been used for testing the metal complexes Ni (II), Co (II) and Cd (II) derived from the ligand (HL); all the details about the redox reactions of these compounds had been obtained. The importance of knowing oxidation and reduction reactions is due to their consideration as the main source of energy for the most biological process, energy productions, photosynthesis to immune responses and the synthesis and breakdown of biomolecules. Therefore, redox reactions are very important in our life.
Keywords: Antibacterial; Corrosion inhibitor; Cyclic voltammatery; DFT and docking study; Quasi-reversible; Thiosemicarbazone.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











References
-
- Liao, X. et al. Corrosion behaviour of copper under chloride-containing thin electrolyte layer. Corros. Sci.53(10), 3289–3298 (2011). - DOI
-
- Cascalheira, A. C., Aeiyach, S., Lacaze, P. C. & Abrantes, L. M. Electrochemical synthesis and redox behaviour of polypyrrole coatings on copper in salicylate aqueous solution. Electrochim. Acta48(17), 2523–2529 (2003). - DOI
-
- Eduok, U., Jossou, E., Tiamiyu, A., Omale, J. & Szpunar, J. Ceria/acrylic polymer microgel composite: Synthesis, characterization, and anticorrosion application for API 5L X70 substrate in chloride-enriched medium. Ind. Eng. Chem. Res.56(19), 5586–5597 (2017). - DOI
-
- Umoren, S. A., Ogbobe, O., Igwe, I. O. & Ebenso, E. E. Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros. Sci.50(7), 1998–2006 (2008). - DOI
-
- Ismayilov, I. T. et al. In inhibition effects of some novel surfactants based on corn oil and diethanolamine on mild steel corrosion in chloride solutions saturated with CO2. Int. J. Thin Films Sci. Technol.2, 91 (2013). - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

