Age dependent susceptibility and immune responses to La Crosse virus infection in non-human primates
- PMID: 40360698
- PMCID: PMC12075599
- DOI: 10.1038/s41598-025-01285-8
Age dependent susceptibility and immune responses to La Crosse virus infection in non-human primates
Abstract
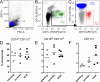
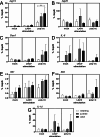
La Crosse virus (LACV) is a primary cause of pediatric viral encephalitis in the United States but rarely causes disease in adults. We tested whether cynomolgus macaques displayed a similar age-dependent susceptibility to LACV. Immune responses from naïve or LACV infected weanling (9-15 months), juvenile (19-23 months) or adult (> 6 years) animals were measured and infected animals were monitored for disease. Naïve weanling animals had fewer dendritic cells in their blood and weaker induction of IFN-stimulated genes (ISG) and chemokines when PBMCs were stimulated in vitro. While no infected animals developed disease, the weaker innate response in naive weanlings correlated with increased viral RNA in plasma from 2 of 3 infected weanlings out to 7 days post infection (dpi). Activated CD8+ T cells and neutralizing antibody proportions were similar amongst all ages. However, CD4+ T cells proportions were increased in young animals relative to adults. This suggests the CD4+ adaptive response in young animals may be bolstering an initially weak innate response to clear virus. Finally, because macaques were resistant to disease, we infected 3 common marmosets intranasally with LACV. Marmoset were selected due to their susceptibility to viral encephalitis. Although no animals showed disease signs, one animal had evidence of infection in the nasal mucosa out to 23 days with associated vacuolization, edema and immune cell infiltration.
Keywords: Age-related susceptibility; Immune response; Interferon stimulated gene; La Crosse virus; Non-human primate; T cell.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Fitzgerald, J. & Livdahl, T. P. Vertical habitat stratification in sympatric and allopatric populations of Aedes hendersoni and Aedes triseriatus (Diptera: Culicidae). J. Med. Entomol.56, 311–319. 10.1093/jme/tjy107 (2019). - PubMed
-
- Koloski, C. W., Drahun, I. & Cassone, B. J. Occurrence of the mosquito Aedes triseriatus (Diptera: Culicidae) beyond its most Northwestern range limits in Manitoba, Canada. J. Med. Entomol.58, 1958–1961. 10.1093/jme/tjab021 (2021). - PubMed
-
- Westby, K. M., Fritzen, C., Paulsen, D., Poindexter, S. & Moncayo, A. C. La Crosse encephalitis virus infection in Field-Collected Aedes albopictus, Aedes Japonicus, and Aedes triseriatus in Tennessee. J. Am. Mosq. Control Assoc.31, 233–241. 10.2987/moco-31-03-233-241.1 (2015). - PubMed
-
- Evans, A. B. & Peterson, W. C. W. K.E. in Encyclopedia of Virology (Fourth Edition) Vol. Fourth Edition (ed Mark Zuckerman Dennis H. Bamford), 654–665 (2021).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

