Metabolic reprogramming of interleukin-17-producing γδ T cells promotes ACC1-mediated de novo lipogenesis under psoriatic conditions
- PMID: 40360755
- PMCID: PMC12116387
- DOI: 10.1038/s42255-025-01276-z
Metabolic reprogramming of interleukin-17-producing γδ T cells promotes ACC1-mediated de novo lipogenesis under psoriatic conditions
Abstract
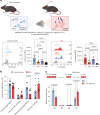
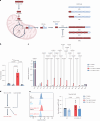
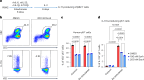
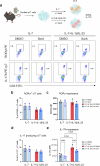
Metabolic reprogramming determines γδ T cell fate during thymic development; however, the metabolic requirements of interleukin (IL)-17A-producing γδ T cells (γδT17 cells) under psoriatic conditions are unclear. Combining high-throughput techniques, including RNA sequencing, SCENITH, proteomics and stable isotope tracing, we demonstrated that psoriatic inflammation caused γδT17 cells to switch toward aerobic glycolysis. Under psoriatic conditions, γδT17 cells upregulated ATP-citrate synthase to convert citrate to acetyl-CoA, linking carbohydrate metabolism and fatty acid synthesis (FAS). Accordingly, we used a pharmacological inhibitor, Soraphen A, which blocks acetyl-CoA carboxylase (ACC), to impair FAS in γδT17 cells, reducing their intracellular lipid stores and ability to produce IL-17A under psoriatic conditions in vitro. We pinpointed the pathogenic role of ACC1 in γδT17 cells in vivo by genetic ablation, ameliorating inflammation in a psoriatic mouse model. Furthermore, ACC inhibition limited human IL-17A-producing γδT17 cells. Targeting ACC1 to attenuate pathogenic γδT17 cell function has important implications for psoriasis management.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: R.J.A. is a scientist and co-founder of GammaOmics, a startup that holds the exclusive license (patent PCT/EP2020/060486) to commercialize and provide services for SCENITH, a technology used in this study. The other authors declare no competing interests.
Figures













References
-
- Lande, R. et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun.5, 5621 (2014) (published correction appears in Nat. Commun.6, 6595 (2015)). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

