Neuroprotective effects of human umbilical cord mesenchymal stem cells (Neuroncell-EX) in a rat model of ischemic stroke are mediated by immunomodulation, blood-brain barrier integrity, angiogenesis, and neurogenesis
- PMID: 40360812
- PMCID: PMC12125091
- DOI: 10.1007/s11626-025-01037-y
Neuroprotective effects of human umbilical cord mesenchymal stem cells (Neuroncell-EX) in a rat model of ischemic stroke are mediated by immunomodulation, blood-brain barrier integrity, angiogenesis, and neurogenesis
Abstract
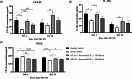
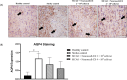
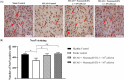
Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) are a potential off-the-shelf product for acute ischemic stroke. This study explored the underlying mechanism of Cytopeutics® hUC-MSCs (Neuroncell-EX) as well as its feasibility and efficacy at two different doses: 2 × 106 cells per rat and 4 × 106 cells/rat in middle cerebral artery occlusion (MCAO) ischemic stroke model for 28 d. Modified neurological severity score (mNSS) and rotarod tests were evaluated at days 1, 4, 7, and 14. Transforming growth factor-beta 1 (TGF-β1), interleukin-1 receptor antagonist (IL-1Ra), and vascular endothelial growth factor (VEGF) were evaluated by enzyme-linked immunosorbent assay (ELISA) at days 4 and 28. Immunohistochemistry expression of aquaporin-4 (AQP4) and neuronal protein marker (NeuN) were performed at days 4 and 28, respectively. Both doses of Neuroncell-EX showed significant lower mNSS scores at days 7 and 14 compared to stroke control. Both Neuroncell-EX groups showed significant longer latency time at day 7, with only 4 × 10⁶ cells/rat group having significant longer time at day 14 than stroke control. At both time points, the 2 × 10⁶ cells/rat group had significantly higher TGF-β1 and IL-1Ra levels, with significantly increased TGF-β1 only observed in 4 × 10⁶ cells/rat group at day 4 compared to stroke control. The VEGF levels were significantly lower at day 4 but then significantly increased at day 28 in both Neuroncell-EX groups than stroke control. AQP4 expression was significantly higher in stroke control compared to healthy control at day 4. Both doses of Neuroncell-EX showed significantly higher NeuN expression compared to stroke control at day 28. There is a weak correlation between TGF-β1 with VEGF and inversely with AQP4. These results suggest that Neuroncell-EX is feasible and effective in promoting functional recovery and neuroprotection in ischemic rats, potentially through immunomodulation, angiogenesis, and neurogenesis mechanisms.
Keywords: Angiogenesis; Immunomodulation; Ischemic stroke; Neurogenesis; Neuroinflammation; hUC-MSCs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics statement: Animal ethics approval was obtained from the Institutional Animal Ethics Committee (Syngene/IAEC/1324–12-2021), Syngene International, Bangalore, India, reported according to ARRIVE and International Committee of Medical Journal Editors (ICMJE) guidelines. Conflict of interest: C.S.P. advises Cytopeutics Sdn Bhd. on regulatory, clinical, and research activities. E.N.A.A.R. and N.N.N.A. have no conflict of interest to declare.
Figures


Similar articles
-
Protective Effects of Transforming Growth Factor-β1 Knockdown in Human Umbilical Cord Mesenchymal Stem Cells against Subarachnoid Hemorrhage in a Rat Model.Cerebrovasc Dis. 2020;49(1):79-87. doi: 10.1159/000505311. Epub 2020 Jan 15. Cerebrovasc Dis. 2020. PMID: 31940632
-
Neuroprotection of Human Umbilical Cord-Derived Mesenchymal Stem Cells (hUC-MSCs) in Alleviating Ischemic Stroke-Induced Brain Injury by Regulating Inflammation and Oxidative Stress.Neurochem Res. 2024 Oct;49(10):2871-2887. doi: 10.1007/s11064-024-04212-x. Epub 2024 Jul 18. Neurochem Res. 2024. PMID: 39026086
-
Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats.Brain Res. 2008 Sep 10;1229:233-48. doi: 10.1016/j.brainres.2008.06.087. Epub 2008 Jul 2. Brain Res. 2008. PMID: 18634757
-
Enhancing the Therapeutic Potential of CCL2-Overexpressing Mesenchymal Stem Cells in Acute Stroke.Int J Mol Sci. 2020 Oct 21;21(20):7795. doi: 10.3390/ijms21207795. Int J Mol Sci. 2020. PMID: 33096826 Free PMC article.
-
Synergistic therapeutic effects of intracerebral transplantation of human modified bone marrow-derived stromal cells (SB623) and voluntary exercise with running wheel in a rat model of ischemic stroke.Stem Cell Res Ther. 2023 Jan 24;14(1):10. doi: 10.1186/s13287-023-03236-4. Stem Cell Res Ther. 2023. PMID: 36691091 Free PMC article.
References
-
- Ahad MA, Kumaran KR, Ning T, Mansor NI, Effendy MA, Damodaran T, Lingam K, Wahab HA, Nordin N, Liao P, Müller CP, Hassan Z (2020) Insights into the neuropathology of cerebral ischemia and its mechanisms. Rev Neurosci 31(5):521–538. 10.1515/revneuro-2019-0099 - PubMed
-
- Battista D, Ferrari CC, Gage FH, Pitossi FJ (2006) Neurogenic niche modulation by activated microglia: transforming growth factor β increases neurogenesis in the adult dentate gyrus. Eur J Neurosci 23(1):83–93. 10.1111/j.1460-9568.2005.04539.x - PubMed
-
- Chen B, Zhang Y, Chen S, Li X, Dong J, Chen W, Tao S, Yang W, Zhang Y (2021) The role of vascular endothelial growth factor in ischemic stroke. Pharmazie 76(4):127–131. 10.1691/ph.2021.1315 - PubMed
-
- Cheng Q, Zhang Z, Zhang S, Yang H, Zhang X, Pan J, Weng L, Sha D, Zhu M, Hu X, Xu Y (2015) Human umbilical cord mesenchymal stem cells protect against ischemic brain injury in mouse by regulating peripheral immunoinflammation. Brain Res 1594:293–304. 10.1016/j.brainres.2014.10.065 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

