Identification of druggable binding sites and small molecules as modulators of TMC1
- PMID: 40360848
- PMCID: PMC12075566
- DOI: 10.1038/s42003-025-07943-x
Identification of druggable binding sites and small molecules as modulators of TMC1
Abstract
Our ability to hear and maintain balance relies on the proper functioning of inner ear sensory hair cells, which translate mechanical stimuli into electrical signals via mechano-electrical transducer (MET) channels, composed of TMC1/2 proteins. However, the therapeutic use of ototoxic drugs, such as aminoglycosides and cisplatin, which can enter hair cells through MET channels, often leads to profound auditory and vestibular dysfunction. To date, our understanding of how small-molecule modulators interact with TMCs remains limited, hampering the discovery of novel drugs. Here, we propose a structure-based drug screening approach, integrating 3D-pharmacophore modeling, molecular dynamics simulations of the TMC1 + CIB2 + TMIE complex, and experimental validation. Our pipeline successfully identified three potential drug-binding sites within the TMC1 pore, phospholipids, and key amino acids involved in the binding of several compounds, as well as FDA-approved drugs that reduced dye uptake in cultured cochlear explants. Our pipeline offers a broad application for discovering modulators for mechanosensitive ion channels.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


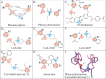


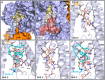
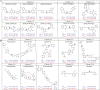

Update of
-
Identification of Druggable Binding Sites and Small Molecules as Modulators of TMC1.bioRxiv [Preprint]. 2024 Dec 20:2024.03.05.583611. doi: 10.1101/2024.03.05.583611. bioRxiv. 2024. Update in: Commun Biol. 2025 May 13;8(1):742. doi: 10.1038/s42003-025-07943-x. PMID: 38826329 Free PMC article. Updated. Preprint.
References
-
- Eileen, D. Noise and hearing loss: a review. J. Sch. Health77, 225–231 (2007). - PubMed
MeSH terms
Substances
Grants and funding
- R01 DC021795/DC/NIDCD NIH HHS/United States
- T32 DC000038/DC/NIDCD NIH HHS/United States
- R01 DC020190/DC/NIDCD NIH HHS/United States
- R01 DC017166/DC/NIDCD NIH HHS/United States
- R01DC020190, R01DC021795, R01DC017166, and T32 DC000038/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
LinkOut - more resources
Full Text Sources
Miscellaneous

