Awareness and Attitudes Regarding Adverse Drug Events and Reporting in South Africa
- PMID: 40360900
- PMCID: PMC12181093
- DOI: 10.1007/s43441-025-00795-x
Awareness and Attitudes Regarding Adverse Drug Events and Reporting in South Africa
Abstract
Background: Reporting of adverse drug events (ADEs), by consumers enhances medication-related risk surveillance, public awareness, and understanding of medicine safety. The aim of this study was to explore adults' awareness of ADEs, attitudes towards reporting and perceptions of their role in reporting ADEs in South Africa.
Method: We conducted a cross-sectional, analytical study in which adults residing in South Africa completed an online questionnaire. The data collected was analysed using both descriptive and inferential statistics.
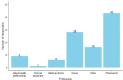
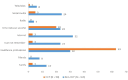
Results: We received responses from 350 participants. Most participants (86.2%, n = 302; N = 350) reported having heard about ADEs and the majority of participants (94.4%, n = 301; N = 319) indicated that reporting of ADEs was important. The Med Safety App was not widely known (17.3%, n = 58; N = 336) while the South African Health Products Regulatory Authority (SAHPRA) was relatively well known (77.4%, n = 260; N = 336). Healthcare providers only educated 55.7% (n = 180; N = 323) of the participants about ADEs and only 50.5% (n = 163; N = 323) of the participants asked their healthcare providers about ADEs. Awareness regarding ADEs was significantly higher (p < 0.001) among healthcare professionals (HCPs) compared to non-healthcare professionals (non-HCP).
Conclusion: Most participants were aware of ADEs and agreed it was important to report ADEs although reporting tools, such as the Med Safety App, were not well known. We recommend awareness campaigns on reporting processes because this could improve consumer reporting of ADEs in South Africa.
Keywords: Adverse drug events; Consumer; Drug side-effects; Pharmacovigilance; Reporting.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: The authors declare no competing interests.
Figures


Similar articles
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review.Cochrane Database Syst Rev. 2018 Apr 17;4(4):CD010842. doi: 10.1002/14651858.CD010842.pub2. Cochrane Database Syst Rev. 2018. PMID: 29664187 Free PMC article.
-
Interventions for the management of abdominal pain in Crohn's disease and inflammatory bowel disease.Cochrane Database Syst Rev. 2021 Nov 29;11(11):CD013531. doi: 10.1002/14651858.CD013531.pub2. Cochrane Database Syst Rev. 2021. PMID: 34844288 Free PMC article.
-
Surveillance for Violent Deaths - National Violent Death Reporting System, 50 States, the District of Columbia, and Puerto Rico, 2022.MMWR Surveill Summ. 2025 Jun 12;74(5):1-42. doi: 10.15585/mmwr.ss7405a1. MMWR Surveill Summ. 2025. PMID: 40493548 Free PMC article.
-
Individual-level interventions to reduce personal exposure to outdoor air pollution and their effects on people with long-term respiratory conditions.Cochrane Database Syst Rev. 2021 Aug 9;8(8):CD013441. doi: 10.1002/14651858.CD013441.pub2. Cochrane Database Syst Rev. 2021. PMID: 34368949 Free PMC article.
References
-
- WHO (World Health Organization). The safety of medicines in public health programmes: pharmacovigilance an essential tool. WHO Press. 2006. Accessed 20 Aug. 2023. https://apps.who.int/iris/handle/10665/43384
-
- Hazell L, Cornelius V, Hannaford P, Shakir S, Avery AJ, Yellow Card Study C. How do patients contribute to signal detection? A retrospective analysis of spontaneous reporting of adverse drug reactions in the UK’s yellow card scheme. Drug Saf. 2013;36(3):199–206. 10.1007/s40264-013-0021-2. - PubMed
-
- ENCePP (European Network of Centres for Pharmacoepidemiology and Pharmacovigilance). Guide on Methodological Standards in Pharmacoepidemiology. Accessed 17 Aug 2023. https://www.encepp.eu/standards_and_guidances/methodologicalGuide7_4.shtml
-
- Harmark L, van Hunsel F, Grundmark B. ADR reporting by the general public: lessons learnt from the Dutch and Swedish systems. Drug Saf. 2015;38(4):337–47. 10.1007/s40264-015-0264-1. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

