31P-MRS saturation transfer for assessing human hepatic ATP synthesis at clinical field strength
- PMID: 40360906
- PMCID: PMC12075714
- DOI: 10.1186/s41747-025-00588-9
31P-MRS saturation transfer for assessing human hepatic ATP synthesis at clinical field strength
Abstract
Background: 31P-magnetic resonance spectroscopy (MRS) saturation transfer (ST) allows for noninvasive investigation of liver energy metabolism by assessing flux rates of adenosine triphosphate (ATP) synthesis. However, this technique has rarely been applied at clinical field strengths because of long examination times and contamination from muscle tissue. Our aim was to establish a new method to robustly assess ATP synthesis using a clinical scanner.
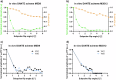
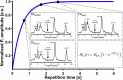
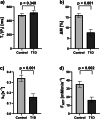
Methods: A prospective single-center study was performed (January 2023-August 2024) within the German Diabetes Study. We established a suitable 31P-MRS ST protocol, tested it in vitro and in vivo and assessed its reproducibility. We assessed the hepatic apparent spin-lattice relaxation time of inorganic phosphate ( ), equilibrium forward rate constant ( ), and forward ATP synthesis rate ( ) in nine control volunteers (CON) (six females) and eight patients (five females) with type 1 diabetes (T1D) and compared differences by ANOVA.
Results: Reproducibility assessment in nine CON, aged 27 ± 4 years (mean ± standard deviation), yielded coefficients of variation for repeated measurements of 7.1% and 21.3% for and , respectively. Group comparison revealed higher hepatic (0.34 ± 0.03 s-1 versus 0.16 ± 0.03 s-1; p = 0.001) and (35.3 ± 3.5 mM/min versus 16.4 ± 3.5 mM/min; p = 0.002) in CON than in T1D, aged 42 ± 15 years, respectively.
Conclusion: This 31P-MRS ST method allowed for robust assessment of hepatic ATP synthesis at clinical field strength and was sensitive enough to detect differences between CON and T1D volunteers.
Relevance statement: Noninvasive methods to investigate hepatic energy metabolism are urgently needed to evaluate liver health while preventing unnecessary biopsies. For broad clinical applicability, the robustness shown by the proposed method at clinical field strength is crucial.
Trial registration: ClinicalTrials.gov: NCT01055093-Prospective study on diabetes mellitus and its complications in newly diagnosed adult patients (GDC), NCT01055093, Registered: 01/22/2010, https://clinicaltrials.gov/study/NCT01055093?term=NCT01055093&rank=1#study-overview .
Key points: The proposed magnetic resonance spectroscopy method calculates hepatic ATP synthesis rates at clinical field strength. The protocol shows acceptable reproducibility and spectra without contamination from muscle. The method can detect differences between participants with type 1 diabetes and controls.
Keywords: Adenosine triphosphate; Diabetes mellitus (type 1); Energy metabolism; Liver; Magnetic resonance spectroscopy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Institutional Review Board approval was obtained. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee (Medical Faculty, Heinrich Heine University, Düsseldorf; ref#4508) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Consent for publication: All participants provided written informed consent. Competing interests: The authors of this manuscript declare relationships with the following companies. MR received fees consulting, lecturing or serving on advisory boards from Astra Zeneca, Boehringer-Ingelheim, Echosens, Eli Lilly, Madrigal, Merck-MSD, and Novo Nordisk and has performed investigator-initiated research with support from Boehringer-Ingelheim, Novo Nordisk, and Nutricia/Danone to the German Diabetes Center (DDZ). The other authors declare that they have no competing interests.
Figures
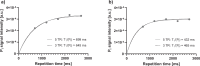

References
-
- Fromenty B, Roden M (2023) Mitochondrial alterations in fatty liver diseases. J Hepatol 78:415–429. 10.1016/j.jhep.2022.09.020 - PubMed
-
- Koliaki C, Szendroedi J, Kaul K et al (2015) Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 21:739–746. 10.1016/j.cmet.2015.04.004 - PubMed
-
- Harrison SA, Bedossa P, Guy CD et al (2024) A phase 3, randomized, controlled trial of Resmetirom in NASH with liver fibrosis. N Engl J Med 390:497–509. 10.1056/NEJMoa2309000 - PubMed
-
- Gancheva S, Kahl S, Pesta D et al (2022) Impaired hepatic mitochondrial capacity in nonalcoholic steatohepatitis associated with type 2 diabetes. Diabetes Care 45:928–937. 10.2337/dc21-1758 - PubMed
-
- Oberhaensli RD, Galloway GJ, Hilton-Jones D et al (1987) The study of human organs by phosphorus-31 topical magnetic resonance spectroscopy. Br J Radiol 60:367–373. 10.1259/0007-1285-60-712-367 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
