An Adult Population Pharmacokinetic Model to Simulate Subcutaneous Administration of a Fixed Dose of Furosemide in Adolescents with Heart Failure and Volume Overload
- PMID: 40360952
- PMCID: PMC12159125
- DOI: 10.1007/s40262-025-01515-2
An Adult Population Pharmacokinetic Model to Simulate Subcutaneous Administration of a Fixed Dose of Furosemide in Adolescents with Heart Failure and Volume Overload
Abstract
Background: Subcutaneous furosemide administered with the On-Body Infusor could be useful in children with heart failure (HF) and congestion due to volume overload, but the appropriate dosing regimen is unknown.
Objective: This study aimed to develop a population pharmacokinetic (popPK) model to determine the subcutaneous furosemide dosing regimen in children with HF who are appropriate for On-Body Infusor use.
Methods: Samples collected from 15 adults with HF who received subcutaneous or intravenous furosemide in a randomized phase II/III study (NCT02329834) were used to develop the popPK model with covariates identified by forward inclusion and backward elimination; validation was by bootstrapping. The model was allometrically scaled from a 70-kg adult body weight to simulate furosemide pharmacokinetics in virtual adolescents aged 12-17 years by weight category (42.5-50.0, > 50-60, and > 60-70 kg) for subcutaneous furosemide 80 mg (30 mg over 1 h then 12.5 mg/h for 4 h).
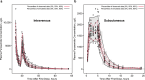
Results: Furosemide pharmacokinetics were best characterized using a two-compartment model with first-order absorption and elimination. After scaling to adolescents in subcutaneous dosing simulations, estimated furosemide clearance was 1.55 mL/min/kg. Estimated exposure (mean area under the plasma concentration-time curve at 24 h) was 16,800 µg⋅h/L in adolescents weighing 42.5-50.0 kg, 14,700 µg⋅h/L in adolescents weighing > 50-60 kg, and 13,000 µg⋅h/L in adolescents weighing > 60-70 kg versus 12,400 µg⋅h/L in adults.
Conclusions: Simulated furosemide exposure was consistent with published values, supporting an 80-mg dose of subcutaneous furosemide (30 mg over the first hour, then 12.5 mg/h for 4 h) for adolescents aged 12-17 years with body weight ≥ 42.5 kg.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This study was sponsored by scPharmaceuticals, Inc. Conflict of Interest: CPH has no conflicts to disclose. HPF is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award 1T32HD104576. EK and JM are employees of and own stock in scPharmaceuticals, Inc. Availability of Data and Material: Data are available upon reasonable request by emailing the corresponding author at christoph.hornik@duke.edu. Code Availability: Not applicable. Author Contributions: CPH developed the analysis plan, performed the analyses, and contributed to the interpretation of the findings. HPF contributed to the interpretation of the findings. EK contributed to the study design and study protocol development. JM contributed to the study conceptualization, study design, and interpretation of findings. All authors contributed to the development and critical review of the manuscript and approved the final version for submission. Ethics Approval: This clinical study was conducted according to the principles of the Declaration of Helsinki. The study protocol was approved by the ethical review committee and institutional review board at the participating site. Consent to Participate: Patients provided written, informed consent before study participation. Consent for Publication: Not applicable.
Figures

References
-
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032. 10.1161/CIR.0000000000001063. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

