Safety Evaluation of Contezolid (MRX-I) Versus Linezolid in Sprague-Dawley Rats
- PMID: 40360967
- PMCID: PMC12185790
- DOI: 10.1007/s40268-025-00504-x
Safety Evaluation of Contezolid (MRX-I) Versus Linezolid in Sprague-Dawley Rats
Abstract
Background & objectives: Contezolid (MRX-I) is a novel ortho-fluorophenyl dihydropyridone developed by MicuRx Pharmaceuticals, Inc. It has been approved for the treatment of drug-resistant Gram-positive bacterial infections with relatively lower toxicity than other oxazolidinones such as linezolid. However, the toxicity profile has not yet been completely revealed. The aim of this study was to disclose the toxicity of contezolid in Sprague-Dawley (SD) rats and compare its toxicity profile with linezolid in a standard 4-week toxicity study.
Methods: In this study, SD rats were orally administered with contezolid at doses of 20, 100, or 200/300 mg/kg/day for 28 consecutive days followed by a 28-day recovery period. Linezolid at doses of 100 or 200 mg/kg/day served as a comparator. Clinical observations, body weight, food consumption, hematology, clinical chemistry, urinalysis, and histopathological examinations were conducted.
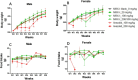
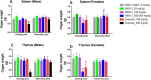
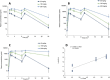
Results: All females in the 200 mg/kg/day linezolid group were subjected to unscheduled death due to myelosuppression within the first 2 weeks. No abnormalities were noted in the 200 mg/kg/day contezolid group, and the dose level was escalated to 300 mg/kg/day from day 15. Myelosuppression or myelosuppression-associated effects were comparable between the 300-mg/kg/day contezolid group and the 100-mg/kg/day linezolid group. The 'no observed adverse effect level' (NOAEL) of contezolid was determined to be 100 mg/kg/day (with an average AUC0-24 h of 268.4 μg*h/mL). At the same dose levels, the toxicity of contezolid was significantly lower than that of linezolid.
Conclusion: These findings demonstrate that contezolid exhibits a favorable safety profile compared with linezolid in this 4-week repeated-dose toxicity study in rats.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This study was sponsored by MicuRx Pharmaceuticals Inc. The funder had no role in the study design, data analysis, or interpretation of results. Conflict of Interest: InnoStar was contracted by MicuRx Pharmaceuticals Inc. to conduct this study. However, the authors declare that this contractual relationship did not influence the study design, data collection, analysis, or interpretation of results. The authors also confirm that they have no financial investments in MicuRx Pharmaceuticals Inc. Ethics Approval: All protocols and procedure were ethically approved and monitored by the Institutional Animal Care and Use Committee (IACUC) in Shanghai Innostar Bio-tech Co., Ltd. (reference number: IACUC-2009-r-008). Consent to Participate: Not applicable. Consent for Publication: Not applicable. Data Availability Statement: All data supporting the findings are included in the article. The detailed datasets and raw records are available from Shanghai Innostar Bio-tech Co., Ltd upon reasonable request. Code Availability Statement: Not applicable. Authors’ Contributions: Conception and study design: YC and YLQ. Data acquisition: NPT and HL. Analysis and interpretation: ML, YMQ, and QML. Drafting of the manuscript: LPW and MH. All authors read and approved the final version of the manuscript.
Figures

Similar articles
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults.Cochrane Database Syst Rev. 2017 Aug 14;8(8):CD012763. doi: 10.1002/14651858.CD012763. Cochrane Database Syst Rev. 2017. PMID: 28806470 Free PMC article.
-
Safety Assessment of Butyric Acid-Rich Triglyceride Oil: A Novel Palatable Formulation of Butyrate for the Pediatric Population.J Appl Toxicol. 2025 Apr;45(4):587-605. doi: 10.1002/jat.4729. Epub 2024 Nov 28. J Appl Toxicol. 2025. PMID: 39609950
-
Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome.Cochrane Database Syst Rev. 2022 Mar 11;3(3):CD013130. doi: 10.1002/14651858.CD013130.pub2. Cochrane Database Syst Rev. 2022. PMID: 35274741 Free PMC article.
-
Intensified versus standard dose infliximab induction therapy for steroid-refractory acute severe ulcerative colitis (PREDICT-UC): an open-label, multicentre, randomised controlled trial.Lancet Gastroenterol Hepatol. 2024 Nov;9(11):981-996. doi: 10.1016/S2468-1253(24)00200-0. Epub 2024 Sep 2. Lancet Gastroenterol Hepatol. 2024. PMID: 39236736 Clinical Trial.
References
-
- Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America. Clin Infect Dis. 2006;42(5):657–68. 10.1086/499819. - PubMed
-
- Wu XJ, Li YF, Zhang J, Zhang YY, Yu JW, Cao GY, et al. Short-term safety, tolerability, and pharmacokinetics of MRX-I, an oxazolidinone antibacterial agent, in healthy Chinese subjects. Clin Ther. 2018;40(2):322-332.e5. 10.1016/j.clinthera.2017.12.017. - PubMed
-
- Shinabarger D. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin Investig Drugs. 1999;8(8):1195–202. 10.1517/13543784.8.8.1195. - PubMed
-
- Falagas ME, Vardakas KZ. Benefit-risk assessment of linezolid for serious gram-positive bacterial infections. Drug Saf. 2008;31(9):753–68. 10.2165/00002018-200831090-00004. - PubMed
-
- Meng J, Zhong DF, Li L, Yuan ZY, Yuan H, Xie C, et al. Metabolism of MRX-I, a novel antibacterial oxazolidinone, in humans: the oxidative ring opening of 2,3-dihydropyridin-4-one catalyzed by non-P450 enzymes. Drug Metab Dispos. 2015;43(5):646–59. 10.1124/dmd.114.061747. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

