Diabetes mellitus aggravates myocardial inflammation and oxidative stress in aortic stenosis: a mechanistic link to HFpEF features
- PMID: 40361188
- PMCID: PMC12070770
- DOI: 10.1186/s12933-025-02748-y
Diabetes mellitus aggravates myocardial inflammation and oxidative stress in aortic stenosis: a mechanistic link to HFpEF features
Abstract
Background: Patients diagnosed with both aortic stenosis (AS) and diabetes mellitus (DM) encounter a distinctive set of challenges due to the interplay between these two conditions. This study aimed to investigate the effects of DM on the left ventricle in AS patients, specifically focusing on the inflammatory response, oxidative stress, and their implications for cardiomyocyte function, titin phosphorylation, and the nitric oxide (NO)-soluble guanylyl cyclase (sGC)-cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) signaling pathway.
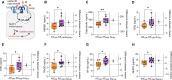
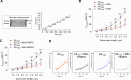
Methods and results: Left ventricular myocardial biopsies (in total: n = 28) were obtained from patients with diabetic AS (n = 11) and compared with those from non-diabetic AS patients (n = 17). Enzyme-linked immunosorbent assay (ELISA) demonstrated significantly elevated levels of pro-inflammatory mediators, including high mobility group box protein 1 (HMGB1) and calprotectin, as well as receptors associated with the inflammatory response, such as Toll-like receptor 2 (TLR2), 4 (TLR4), and receptor for advanced glycation endproducts (RAGE). These were correlated with an enhanced NOD-like receptor protein 3 (NLRP3) inflammasome and the release of interleukins (IL) 1, 6, and 18 in diabetic AS patients compared to their non-diabetic counterparts. Additionally, in the diabetic AS cohort, there was an increase in oxidative stress markers (hydrogen peroxide (H2O2), 3-nitrotyrosine, lipid peroxidation (LPO), oxidative glutathione (GSSG)/reduced glutathione (GSH) ratio) within the myocardium and mitochondria, accompanied by impaired NO-sGC-cGMP-PKG signaling, decreased titin phosphorylation, and increased passive stiffness (Fpassive) of cardiomyocytes relative to non-diabetic AS patients. In vitro anti-inflammatory treatment with an IL-6 inhibitor and antioxidant treatment with GSH effectively normalized the elevated Fpassive observed in AS patients with DM to levels comparable to the non-diabetic group. Furthermore, treatment with PKG and the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin also resulted in a reduction of Fpassive in cardiomyocytes from diabetic AS patients, although not to the levels observed in non-diabetic AS patients.
Conclusion: DM exacerbates inflammation and oxidative stress in AS patients, leading to impaired NO-sGC-cGMP-PKG signaling and increased cardiomyocyte Fpassive. These conditions are reminiscent of the pathophysiology of heart failure with preserved ejection fraction (HFpEF). These alterations can be ameliorated through anti-inflammatory and antioxidant therapies, indicating potential therapeutic strategies for diabetic patients suffering from AS.
Keywords: Aortic stenosis; Cardiomyocyte Fpassive; Diabetes mellitus; Heart failure with preserved ejection fraction; Inflammation; Oxidative stress; Protein kinase G; Titin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of ULS São João Hospital (Ref 109/2022) following the Declaration of Helsinki. Informed consent was obtained from all patients. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests. P.F. is the founder and CEO of Pharmahungary Group, a group of R&D companies. Francesco Paneni and Era Gorica manage the collection “Cardiometabolic HFpEF with focus on type 2 diabetes mellitus” and the co-author Francesco Paneni is also an Associate Editor of Cardiovascular Diabetology. They haven’t been involved in the peer review of this article.
Figures




References
-
- Kontogeorgos SM, Rosengren A, Zverkova Sandstrom T, Lindgren M. Progression of aortic stenosis in patients with diabetes mellitus type 2 compared with matched controls. Eur Heart J. 2024;45:ehae666-1798.
MeSH terms
Substances
Grants and funding
- MED1814, M2401/Hector Stiftung
- IF-023-22/Innovation Forum program of the Medical Faculty, RUB
- F/17/23/Deutsche Herzstiftung
- RRF-2.3.1-21-2022-00003/Else Kröner-Fresenius-Stiftung
- 739593/EU's Horizon 2020 research and innovation program
- 739593/EU's Horizon 2020 research and innovation program
- FK134751/National Research, Development and Innovation Office (NKFIH) of Hungary
- TKP2021-EGA-23/Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund
- HA 7512/2-4/Deutsche Forschungsgemeinschaft
LinkOut - more resources
Full Text Sources
Medical
Research Materials

