Research on policy mechanisms to address funding bias and conflicts of interest in biomedical research: a scoping review
- PMID: 40361202
- PMCID: PMC12076912
- DOI: 10.1186/s41073-025-00164-0
Research on policy mechanisms to address funding bias and conflicts of interest in biomedical research: a scoping review
Abstract
Background: Industry funding and author conflicts of interest (COI) have been consistently shown to introduce bias into agenda-setting and results-reporting in biomedical research. Accordingly, maintaining public trust, diminishing patient harm, and securing the integrity of the biomedical research enterprise are critical policy priorities. In this context, a coordinated and methodical research effort is required to effectively identify which policy interventions are most likely to mitigate against the risks of funding bias. Subsequently this scoping review aims to identify and synthesize the available research on policy mechanisms designed to address funding bias and COI in biomedical research.
Methods: We searched PubMed for peer-reviewed, empirical analyses of policy mechanisms designed to address industry sponsorship of research studies, author industry affiliation, and author COI at any stage of the biomedical research process and published between January 2009 and 28 August 2023. The review identified literature conducting five primary analysis types: (1) surveys of COI policies, (2) disclosure compliance analyses, (3) disclosure concordance analyses, (4) COI policy effects analyses, and (5) studies of policy perceptions and contexts. Most available research is devoted to evaluating the prevalence, nature, and effects of author COI disclosure policies.
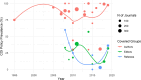
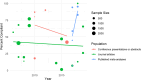
Results: Six thousand three hundreds eighty five articles were screened, and 81 studies were included. Studies were conducted in 11 geographic regions, with studies of international scope being the most common. Most available research is devoted to evaluating the prevalence, nature, and effects of author COI disclosure policies. This evidence demonstrates that while disclosure policies are pervasive, those policies are not consistently designed, implemented, or enforced. The available evidence also indicates that COI disclosure policies are not particularly effective in mitigating risk of bias or subsequent negative externalities.
Conclusions: The results of this review indicate that the COI policy landscape could benefit from a significant shift in the research agenda. The available literature predominantly focuses on a single policy intervention-author disclosure requirements. As a result, new lines of research are needed to establish a more robust evidence-based policy landscape. There is a particular need for implementation research, greater attention to the structural conditions that create COI, and evaluation of policy mechanisms other than disclosure.
Keywords: Competing interests; Conflicts of interest; Funding bias; Meta-research; Research integrity policy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: SSG has received grant funding from NIGMS. JBB has received grant funding from NIGMS, NSF, IC2, and Blue Cross Blue Sheild, royalties form Cengage Publishing, and consulting fees from The Aslan Group and BrainCheck. JFR has received grant funding from NIGMS, NLM, NIA, NIAD, NIMH, Texas Child Mental Health Care Consortium, Michal & Susan Dell Foundation, Texas Alzheimer’s Research and Care Consortium, Austin Public Health, and the Health Care Cost Institute. ZPM has received grant funding from NIGMS. LB’s institution receives consulting fees for her work as a Senior Research Integrity Editor at Cochrane. QG, NS, and JSE report that they have no conflicts of interest.
Figures


References
-
- Graham SS, Karnes MS, Jensen JT, Sharma N, Barbour JB, Majdik ZP, et al. Evidence for stratified conflicts of interest policies in research contexts: a methodological review. BMJ Open. 2022;12(9):e063501. Available from: https://bmjopen.bmj.com/content/12/9/e063501. Cited 2022 Nov 22. - PMC - PubMed
-
- Fabbri A, Lai A, Grundy Q, Bero LA. The Influence of Industry Sponsorship on the Research Agenda: A Scoping Review. Am J Public Health. 2018;108(11):e9–16. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6187765/. Cited 2024 Aug 5. - PMC - PubMed
-
- Bero LA. Tobacco industry manipulation of research. Public Health Rep. 2005;120(2):200–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1497700/. Cited 2024 Aug 17. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

