Non-typhoidal Salmonella transmission reservoirs in Sub-Saharan Africa: a genomic assessment from a one health perspective
- PMID: 40361223
- PMCID: PMC12070793
- DOI: 10.1186/s13756-025-01561-2
Non-typhoidal Salmonella transmission reservoirs in Sub-Saharan Africa: a genomic assessment from a one health perspective
Abstract
Background: In sub-Saharan Africa, invasive non-typhoidal Salmonella disease, characterized by bloodstream infections with high mortality rates, poses a significant public health burden. In Africa, Salmonella enterica, which are typically livestock- associated pathogens in industrialised countries, have genetically evolved and anthroponotic transmission has been proposed for S. Typhimurium ST313. In this study, we investigated the hypothesis of an exclusively anthroponotic transmission reservoir of Salmonella enterica ST313 and aimed to identify reservoirs for other Salmonella spp., shedding light on their occurrence in different ecological niches.
Methods: This study used a One Health approach and Salmonella were isolated from humans, livestock and the environment, in Tanzania and in Ghana. Salmonella spp. were identified by biochemical methods and antibiotic susceptibility was tested. Isolates were subjected to whole genome sequencing.
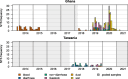
Results: Out of 9,086 collected samples, 222 Salmonella enterica were identified comprising 58 serovars. The highest level of antimicrobial resistance was found in humans with emerging fluroquinolone resistance and multidrug resistance being highest in isolates from blood cultures (24%, n/N = 11/46). For the invasive strains, the sequence types S. Typhimurium ST313 and ST19 were most common and ST313 was associated with multidrug resistance, followed by S. Enteritidis ST11 and ST147 and S. Dublin ST10. An overlap of sequence types amongst human-livestock and human-environmental strains was detected for S. Typhimurium ST19 but not found for ST313 and the two serovars Dublin and Enteritidis.
Conclusions: Our study adds further evidence of S. Typhimurium ST313 being restricted to a human reservoir and linked to multidrug resistance. Additionally, our study provides comprehensive insights into Salmonella genetic diversity and distribution among humans, animals and the environment in Ghana and in Tanzania. This sheds light on other potential reservoirs for infections, all of which show antimicrobial resistance. Further research into stool carriage is warranted, encompassing patients with invasive disease and those with and without diarrhoea, to identify transmission reservoirs in particular for invasive disease-causing strains. These findings underscore the need for integrated One Health approaches to effectively monitor and manage salmonellosis and mitigate public health risks. Continued research into the spread of Salmonella spp. and its evolution is crucial for targeted interventions and disease control.
Keywords: Salmonella enterica; Drug resistance; Human-animal-environmental interface; Molecular epidemiology; Pathogen reservoirs; Tropical Africa.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: In Ghana, The Committee on Human Research, Publications and Ethics, School of Medical Science, Kwame Nkrumah University of Science and Technology in Kumasi, Ghana, approved this study (No. CHRPE/AP/674/19). In Tanzania, ethical approval was obtained from the Tanzania Medical Research Coordinating Committee (MRCC) hosted at the National Institute for Medical Research (NIMR/HQ/R.8a/Vol-IX/2909). In Germany approval was granted by the Ethikkommission der Ärztekammer Hamburg (No. PV5664). Study participants were informed about the purpose of this study and the study procedures. Written informed consent was obtained before enrolment from the child’s parent or guardian. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Kariuki S, Mbae C, Van Puyvelde S, Onsare R, Kavai S, Wairimu C, et al. High relatedness of invasive multi-drug resistant non-typhoidal Salmonella genotypes among patients and asymptomatic carriers in endemic informal settlements in Kenya. PLoS Negl Trop Dis. 2020;14(8): e0008440. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

