Lenalidomide Efficacy in Patients with MDS and Del-5q: Real-World Data from the Hellenic (Greek) National Myelodysplastic & Hypoplastic Syndromes Registry (EAKMYS)
- PMID: 40361316
- PMCID: PMC12071050
- DOI: 10.3390/cancers17091388
Lenalidomide Efficacy in Patients with MDS and Del-5q: Real-World Data from the Hellenic (Greek) National Myelodysplastic & Hypoplastic Syndromes Registry (EAKMYS)
Abstract
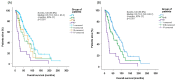
Background-Objectives: Although considered standard of care for patients with low-/intermediate-1 risk MDS and isolated del(5q), lenalidomide is not widely used in patients exhibiting additional cytogenetic abnormalities, on top of del(5)q. The aim of this study was to provide real-world evidence for the efficacy of lenalidomide in patients with del(5q), with or without additional cytogenetic abnormalities. Methods: Patients with MDS exhibiting del(5q) in the Greek National Myelodysplastic Syndromes Registry were analyzed if they had received at least one lenalidomide dose and detailed response assessment/follow-up was available. Results: Among 238 patients analyzed, 153 (64.3%) had del(5q) syndrome (Group-I), 34 (14.3%) had an isolated del(5q) abnormality but were not 5q- syndrome (Group-II), 26 (10.9%) had del(5q) plus only one additional cytogenetic abnormality (Group-III), and 25 (10.5%) had del(5q) plus >1 additional abnormality (Group-IV). Among 218 (91.6%) evaluable patients, a major response was achieved by 146 (67.0%) patients, 114/146 (78.1%) in Group-I, 18/31 (58.1%) in Group-II, 10/20 (50.0%) in Group-III, and 4/21 (19.0%) in Group-IV. Overall, hematological response was seen in 177/218 (81.2%) patients, even among those with an excess of bone marrow blasts/frank acute myeloid leukemia. Duration of response was comparable between the four patient groups. A complete cytogenetic response was achieved by 38.0% overall, more commonly in Group-I (42.3%) and -III (35.7%). Transfusion-independent patients and those with a higher MCV or lower marrow blast cells at baseline had a higher probability of achieving a major response. With multivariate analysis, factors associated with overall survival were age, performance status, transfusion dependence, and marrow blast cell percentage at treatment start, as well as time from initial diagnosis to lenalidomide start. Conclusions: Lenalidomide was highly effective in patients with the del(5)q syndrome and also in those with isolated del(5)q, other than del(5)q syndrome, or those exhibiting del(5)q plus only one additional cytogenetic abnormality, not affecting chromosome 7.
Keywords: del(5)q; lenalidomide; myelodysplastic syndromes; prognosis; real-world-data; treatment.
Conflict of interest statement
Since this study represents an analysis of Registry data regarding real-world experience from the use of an approved drug the authors had not designed any prospective analysis at the time of administration of lenalidomide. Thus, all the authors declare that they have no conflicts of interest, related to this retrospective study. Moreover, there is no intervention by any pharmaceutical company or at any aspect facilitation or financial support in data retrieving and processing and in manuscript preparation, related to this study.
Figures

References
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
-
- Arcioni F., Roncadori A., Di Battista V., Tura S., Covezzoli A., Cundari S., Mecucci C., MORE Study Centres Lenalidomide treatment of myelodysplastic syndromes with chromosome 5q deletion: Results from the National Registry of the Italian Drug Agency. Eur. J. Haematol. 2018;101:78–85. doi: 10.1111/ejh.13067. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

