Risk Factors for Intestinal and Extraintestinal Cancers in Inflammatory Bowel Disease: A Retrospective Single-Center Cohort Study
- PMID: 40361323
- PMCID: PMC12071157
- DOI: 10.3390/cancers17091396
Risk Factors for Intestinal and Extraintestinal Cancers in Inflammatory Bowel Disease: A Retrospective Single-Center Cohort Study
Abstract
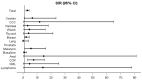
Background/Objectives: Patients with inflammatory bowel disease (IBD) face an increased risk of developing intestinal and extraintestinal cancers. This retrospective single-center study aimed to quantify cancer risk and identify potential risk factors associated with cancer in IBD patients. Methods: The epidemiological data, disease characteristics, treatment regimens, and occurrences of cancer following IBD diagnosis were collected between January 2021 and February 2022. Hazard ratios (HRs) and standardized incidence ratios (SIRs) were estimated. Results: 560 IBD patients were included; 37 patients developed cancer, with 5 patients developing two distinct cancers, resulting in 42 cancers overall. This translated into a twofold increased risk of developing any cancer compared to the general population (SIR 1.94, 95% CI 1.4-2.6). Colorectal (CRC, 29%), skin (19%), and breast cancer (17%) were the most common malignancies. Female patients showed an increased risk for all cancers (SIR 3.1, 95% CI 2.06-4.3), melanoma (SIR 5.6, 95% CI 1.14-16.2), and CRC (SIR 7.5, 95% CI 3-15.4). Conversely, male patients exhibited a significantly increased risk of lymphoma (SIR 26.2, 95% CI 3.2-95.7). Young age at IBD diagnosis and the use of immunomodulators, whether as monotherapy or in combination with biologics, were associated with an increased risk of cancer. Conclusions: The risk of CRC and lymphoma in IBD patients may be higher than previously reported, potentially due to the increasing use of combination therapy. Cancer risk in IBD should be regularly assessed and personalized throughout the disease course.
Keywords: Crohn’s disease (CD); biologics; cancers; immunomodulators; inflammatory bowel disease (IBD); ulcerative colitis (UC).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Beaugerie L., Brousse N., Bouvier A.M., Colombel J.F., Lémann M., Cosnes J., Hébuterne X., Cortot A., Bouhnik Y., Gendre J.P., et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: A prospective observational cohort study. Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources

