Chemotherapy-Free Treatment with Radiotherapy and Immunotherapy for Locally Advanced Non-Small Cell Lung Cancer
- PMID: 40361451
- PMCID: PMC12071140
- DOI: 10.3390/cancers17091524
Chemotherapy-Free Treatment with Radiotherapy and Immunotherapy for Locally Advanced Non-Small Cell Lung Cancer
Abstract
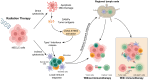
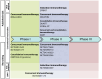
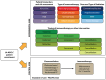
Background: Concurrent chemoradiotherapy (CRT) followed by immunotherapy is a standard treatment for locally advanced non-small cell lung cancer (LA-NSCLC), yet many patients are ineligible due to treatment-related toxicity or poor functional status. Chemotherapy-free approaches using radiotherapy (RT) and immunotherapy may offer a safer and equally effective alternative in select patient populations. Methods: A comprehensive literature review was conducted using PubMed, Google Scholar, and relevant conference proceedings focusing on trials between 2000 and 2024. Studies investigating chemotherapy-free regimens combining RT and immunotherapy in LA-NSCLC were analyzed, with emphasis on clinical outcomes, biomarker use, treatment sequencing, radiation dose/fractionation, and safety. Results: Multiple Phase I/II trials reported promising efficacy with one-year progression-free survival (PFS) ranging from 39% to 76%. Toxicity was generally acceptable, though higher-grade adverse events were more frequent in older, frail populations. Trials integrating PD-L1 expression, tumor mutational burden (TMB), and circulating tumor DNA (ctDNA) showed potential for improved patient stratification. Variation in immunotherapy timing (induction, concurrent, or consolidation) and radiation schedules highlight the need for optimization. Conclusions: Chemotherapy-free regimens represent a promising treatment strategy for patients with LA-NSCLC, especially those that are ineligible for standard CRT. Biomarker-driven patient selection and the rational integration of RT and immunotherapy are critical to improving outcomes. Randomized trials are warranted to establish the efficacy and safety of these emerging approaches.
Keywords: LA-NSCLC; chemotherapy-free; immunotherapy; radiation therapy.
Conflict of interest statement
M.Z.O., A.D., J.Y., and A.R. declare no conflicts of interest. B.H. received grants from Boehringer Ingelheim, Astra Zeneca, Merck, BMS, Advaxis, Amgen, AbbVie, Daiichi, Pfizer, GSK, Beigene, Janssen, Black Diamond Therapeutics, Forward Pharma, Numab, Arrivent; receipient of consulting fees from Astra Zeneca, Boehringer Ingelheim, Apollomics, Janssen, Takeda, Merck, BMS, Genentech, Pfizer, Eli Lilly, Arcus, Merus, Daiichi, Precede; and participated on the Advisory Board of BMS, TPT, Apollomics, eFECTOR, and City of Hope. A.R.F. received fees as a speaker from Astra Zeneca, MSD, Roche, Ipsen; fees for advisory role from Astra Zeneca, Roche; and research funding from Astra Zeneca and also participated in sponsored research from Astra Zeneca, Roche, and MSD. S.H.L. received grant funding from Beyond Spring Pharmaceuticals, Hitachi Chemical Diagnostics, and IntraOp Corporation and serves on the advisory board for Beyond Spring Pharmaceuticals, STCube Pharmaceuticals, and AstraZeneca; he is also a consultant for XRAD Therapeutics and is cofounder of Scenexo, Inc. C.B.S. served in a leadership or fiduciary role for the American Society for Radiation Oncology, Proton Collaborative Group, American Radium Society, and the Particle Therapy Co-Operative Group. N.O. received institutional research funding from Merck, Reflexion Medical and AstraZeneca and received consulting fees for Genentech and Merck. The funders had no role in the analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Spigel D.R., Faivre-Finn C., Gray J.E., Vicente D., Planchard D., Paz-Ares L., Vansteenkiste J.F., Garassino M.C., Hui R., Quantin X., et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022;40:1301–1311. doi: 10.1200/JCO.21.01308. - DOI - PMC - PubMed
-
- Bradley J.D., Sugawara S., Lee K.H.H., Ostoros G., Demirkazik A., Zemanova M., Sriuranpong V., Gelatti A., Menezes J., Zurawski B., et al. LBA1 Durvalumab in Combination with Chemoradiotherapy for Patients with Unresectable Stage III NSCLC: Final Results from PACIFIC-2. ESMO Open. 2024;9:102986. doi: 10.1016/j.esmoop.2024.102986. - DOI
-
- Peters S., Tan D.S.W., Gerber D.E., Urbanic J., Ramalingam S.S., Yu J., Xing L., Rittmeyer A., Ciuleanu T., Menezes J., et al. CheckMate 73L: Phase 3 Study Comparing Nivolumab (N) + Concurrent Chemoradiotherapy (CCRT) Followed by N_Ipilimumab (I) v CCRT Followed by Durvalumab (D) for Previously Untreated, Locally Advanced Stage (Stg) III NSCLC. Ann. Oncol. 2024;24:100808. doi: 10.1016/j.iotech.2024.100808. - DOI
-
- Eichkorn T., Bozorgmehr F., Regnery S., Dinges L.A., Kudak A., Bougatf N., Weber D., Christopoulos P., Muley T., Kobinger S., et al. Consolidation Immunotherapy After Platinum-Based Chemoradiotherapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer—Cross-Sectional Study of Eligibility and Administration Rates. Front. Oncol. 2020;10:586449. doi: 10.3389/fonc.2020.586449. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

