Clinical, Sociodemographic, and Facility-Related Factors Influencing HER2-Targeted Therapy in Metastatic Hormone Receptor-Negative, HER2-Positive Breast Cancer
- PMID: 40361505
- PMCID: PMC12072055
- DOI: 10.3390/cancers17091579
Clinical, Sociodemographic, and Facility-Related Factors Influencing HER2-Targeted Therapy in Metastatic Hormone Receptor-Negative, HER2-Positive Breast Cancer
Abstract
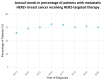
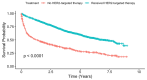
Background/Objectives: The use of HER2-targeted therapies has significantly improved survival outcomes in metastatic hormone receptor-negative, HER2-positive (HR-/HER2+) breast cancer. However, factors influencing their adoption remain unclear. This study examines clinical, sociodemographic, and facility-related determinants of HER2-targeted therapy utilization in metastatic HR-/HER2+ breast cancer. Methods: We conducted a retrospective cohort study of metastatic HR-/HER2+ breast cancer patients from the NCDB (2013-2020), categorizing them into HER2-targeted therapy recipients and non-recipients. Patients with missing key variables were excluded. Time periods were divided as pre-2015, 2016-2018, and 2019-2020 to reflect evolving treatment availability and uptake in the United States. Univariable and multivariable logistic regression identified factors associated with HER2-targeted therapy use. Cox proportional hazards regression and log-rank tests assessed overall survival. Results: Among 3060 metastatic HR-/HER2+ breast cancer patients, 2318 (75.8%) received HER2-targeted therapy. HER2-targeted therapy utilization increased from 64.6% in 2013 to 80.9% in 2016, marking an early period of rapid uptake. Usage remained consistently high from 2016 to 2018, followed by a slight decline and stabilization around 75% from 2019 to 2020. Factors positively associated with therapy use included diagnosis in 2016-2018 (OR 1.93, p < 0.001) and 2019-2020 (1.88, p < 0.001), private insurance (OR 1.76, p < 0.001), and treatment at academic facilities (OR 1.39, p = 0.031). Reduced likelihood of therapy use was observed in patients aged 71+ (OR 0.52, p < 0.001), Black race (OR 0.78, p = 0.018), Medicare insurance (OR 0.64, p < 0.001), and treatment at rural facilities (OR 0.59, p = 0.022). HER2-targeted therapy was associated with significantly improved survival (median 5.08 vs. 1.27 years, log-rank p < 0.001) and lower mortality risk (HR 0.52, p < 0.001). Conclusions: The adoption of HER2-targeted therapy has increased in recent years, yet disparities persist in access and utilization. Our findings highlight the need to address sociodemographic and facility-related barriers to ensure equitable treatment and improved survival outcomes for all patients.
Keywords: HER2-targeted therapy; metastatic HR−/HER2+ breast cancer; survival outcomes; treatment disparities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Exman P., Tolaney S.M. HER2-positive metastatic breast cancer: A comprehensive review. Clin. Adv. Hematol. Oncol. 2021;19:40–50. - PubMed
-
- Swain S.M., Miles D., Kim S.B., Im Y.H., Im S.A., Semiglazov V., Ciruelos E., Schneeweiss A., Loi S., Monturus E., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

