Antiviral Activity of 1-Deoxynojirimycin Extracts of Mulberry Leaves Against Porcine Epidemic Diarrhea Virus
- PMID: 40362022
- PMCID: PMC12071020
- DOI: 10.3390/ani15091207
Antiviral Activity of 1-Deoxynojirimycin Extracts of Mulberry Leaves Against Porcine Epidemic Diarrhea Virus
Abstract
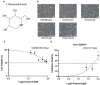
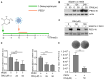
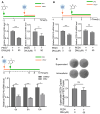
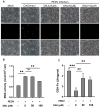
Porcine epidemic diarrhea virus (PEDV), a highly infectious alphacoronavirus, has resulted in substantial economic losses within the global swine industry. Existing vaccines and therapeutic agents have proven inadequate in effectively preventing and controlling PEDV. Natural compounds offer distinct advantages in antiviral research due to their abundant availability, diverse biological activities, and low toxicity. In this study, the antiviral properties of the naturally occurring alkaloid 1-deoxynojirimycin (DNJ) against PEDV were examined. The CC50 of DNJ was determined to be 912.5 μM through experimental analysis on Vero-E6 cells. DNJ demonstrated an inhibitory effect on PEDV activity, with a 50% inhibitory concentration (IC50) of 57.76 μM. The compound primarily inhibited PEDV proliferation during the viral life cycle stages of attachment and replication. Moreover, DNJ mitigated the production of reactive oxygen species (ROS) and inflammation associated with PEDV infection. Computational docking predictions suggest that the viral non-structural proteins include Nsp12, Nsp14, and Nsp16 may serve as potential targets for DNJ. Consequently, DNJ represents a promising candidate for the development of novel therapeutic agents against PEDV.
Keywords: 1-deoxynojirimycin (DNJ); PEDV; antiviral activity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

