Therapeutic Potential of Local and Systemic Adipose-Derived Mesenchymal Stem Cell Injections in a Rat Model of Experimental Periodontitis: Implications for Cardiac Function
- PMID: 40362223
- PMCID: PMC12071214
- DOI: 10.3390/ijms26093984
Therapeutic Potential of Local and Systemic Adipose-Derived Mesenchymal Stem Cell Injections in a Rat Model of Experimental Periodontitis: Implications for Cardiac Function
Abstract
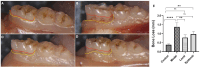
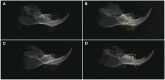
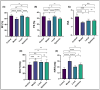
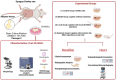
Periodontitis is a common inflammatory disease that not only damages periodontal tissues but also induces systemic effects, including cardiac dysfunction. Mesenchymal stem cells (MSCs) offer regenerative potential due to their ability to differentiate, modulate immune responses, and secrete anti-inflammatory factors. However, the relative efficacy of local versus systemic MSC administration remains unclear. This study evaluated the therapeutic effects of adipose-derived MSCs (AD-MSCs) in a rat model of experimental periodontitis, comparing local and systemic administration. AD-MSCs were characterized based on morphology, surface marker expression, and differentiation potential. Ligature-induced periodontitis was established over 60 days, after which AD-MSCs (1 × 106 cells) were administered either supraperiosteally (local group) or intravenously (systemic group). Periodontal regeneration was assessed through clinical, radiographic, and histopathological analyses, while cardiac function was evaluated using echocardiography and histopathological examinations. Results demonstrated that local AD-MSC administration provided superior therapeutic benefits compared to systemic delivery. Locally administered cells significantly enhanced bone regeneration, reduced inflammation, and improved periodontal tissue architecture. In contrast, systemic administration offered moderate benefits but was less effective in restoring periodontal integrity. Similarly, in the heart, local treatment resulted in greater improvements in systolic function, as indicated by enhanced ejection fraction and fractional shortening, along with reduced myocardial fibrosis. Although systemic administration also provided cardioprotective effects, diastolic dysfunction persisted in both treatment groups. In conclusion, local AD-MSC administration proved more effective in regenerating periodontal tissues and mitigating cardiac dysfunction, highlighting its potential as an optimized therapeutic strategy for periodontitis and its systemic complications.
Keywords: H&E staining; Masson’s trichrome staining; adipose-derived mesenchymal stem cells; cardiac dysfunction; echocardiography; myocardial fibrosis; periodontitis; radiography.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Sanz M., Ceriello A., Buysschaert M., Chapple I., Demmer R.T., Graziani F., Herrera D., Jepsen S., Lione L., Madianos P. Scientific Evidence on the Links between Periodontal Diseases and Diabetes: Consensus Report and Guidelines of the Joint Workshop on Periodontal Diseases and Diabetes by the International Diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018;137:231–241. doi: 10.1016/j.diabres.2017.12.001. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

