Dibromo-Edaravone Induces Anti-Erythroleukemia Effects via the JAK2-STAT3 Signaling Pathway
- PMID: 40362240
- PMCID: PMC12071273
- DOI: 10.3390/ijms26094000
Dibromo-Edaravone Induces Anti-Erythroleukemia Effects via the JAK2-STAT3 Signaling Pathway
Abstract
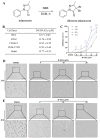
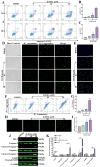
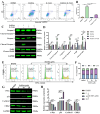
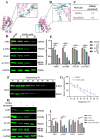
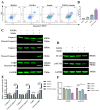
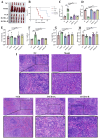
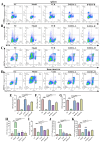
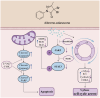
Acute erythroid leukemia (AEL) is a rare and aggressive hematological malignancy managed with chemotherapy, targeted therapies, and stem cell transplantation. However, these treatments often suffer from limitations such as refractoriness, high toxicity, recurrence, and drug resistance, underscoring the urgent need for novel therapeutic approaches. Dibromo-edaravone (D-EDA) is a synthetic derivative of edaravone (EDA) with unreported anti-leukemic properties. In this study, D-EDA demonstrated potent cytotoxicity against HEL cells with an IC50 value of 8.17 ± 0.43 μM using an MTT assay. Morphological analysis via inverted microscopy revealed reductions in cell number and signs of cellular crumpling and fragmentation. Flow cytometry analysis, Hoechst 33258 staining, Giemsa staining, a JC-1 assay, and a reactive oxygen species (ROS) assay showed that D-EDA induced apoptosis in HEL cells. Furthermore, D-EDA induced S-phase cell cycle arrest. Western blot analysis showed significant upregulation of key apoptosis-related proteins, including cleaved caspase-9, cleaved caspase-3, and cleaved poly ADP-ribose polymerase (PARP), alongside a reduction in Bcl-2 expression. Additionally, oncogenic markers such as c-Myc, CyclinA2, and CDK2 were downregulated, while the cell cycle inhibitor p21 was upregulated. Mechanistic studies involving molecular docking, a cellular thermal shift assay (CETSA), the caspase inhibitor Z-VAD-FMK, JAK2 inhibitor Ruxolitinib, and STAT3 inhibitor Stattic revealed that D-EDA activates the caspase cascade and inhibits the JAK2-STAT3 signaling pathway in HEL cells. In vivo, D-EDA improved spleen structure, increased the hemolysis ratio, and extended survival in a mouse model of acute erythroleukemia. In conclusion, D-EDA induces apoptosis via the caspase cascade and JAK2-STAT3 signaling pathway, demonstrating significant anti-leukemia effects in vitro and in vivo. Thus, D-EDA may be developed as a potential therapeutic agent for acute erythroleukemia.
Keywords: JAK2-STAT3; apoptosis; cell cycle; dibromo-edaravone; erythroleukemia.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
Similar articles
-
Mechanism of Shuangyang Houbitong granules against acute erythroleukemia through PI3K/AKT and JAK/STAT signaling pathways.J Ethnopharmacol. 2025 Jun 26;350:119979. doi: 10.1016/j.jep.2025.119979. Epub 2025 May 15. J Ethnopharmacol. 2025. PMID: 40381820
-
JAK2 regulation by licochalcone H inhibits the cell growth and induces apoptosis in oral squamous cell carcinoma.Phytomedicine. 2019 Jan;52:60-69. doi: 10.1016/j.phymed.2018.09.180. Epub 2018 Sep 18. Phytomedicine. 2019. PMID: 30599913
-
A natural acylphloroglucinol exerts anti-erythroleukemia effects via targeting STAT3 and p38-MAPK, and inhibiting PI3K/AKT/mTOR signaling pathway.Biomed Pharmacother. 2024 Nov;180:117424. doi: 10.1016/j.biopha.2024.117424. Epub 2024 Sep 19. Biomed Pharmacother. 2024. PMID: 39303451
-
Edaravone attenuates myocyte apoptosis through the JAK2/STAT3 pathway in acute myocardial infarction.Free Radic Res. 2020 May;54(5):351-359. doi: 10.1080/10715762.2020.1772469. Epub 2020 Jun 16. Free Radic Res. 2020. PMID: 32543312
-
Nootkatone Derivative Nootkatone-(E)-2-iodobenzoyl hydrazone Promotes Megakaryocytic Differentiation in Erythroleukemia by Targeting JAK2 and Enhancing JAK2/STAT3 and PKCδ/MAPK Crosstalk.Cells. 2024 Dec 26;14(1):10. doi: 10.3390/cells14010010. Cells. 2024. PMID: 39791711 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

