FAM20B-Catalyzed Glycosylation Regulates the Chondrogenic and Osteogenic Differentiation of the Embryonic Condyle by Controlling IHH Diffusion and Release
- PMID: 40362273
- PMCID: PMC12071210
- DOI: 10.3390/ijms26094033
FAM20B-Catalyzed Glycosylation Regulates the Chondrogenic and Osteogenic Differentiation of the Embryonic Condyle by Controlling IHH Diffusion and Release
Abstract
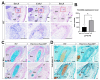
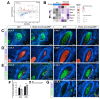
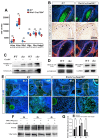
Although the roles of proteoglycans (PGs) have been well documented in the development and homeostasis of the temporomandibular joint (TMJ), how the glycosaminoglycan (GAG) chains of PGs contribute to TMJ chondrogenesis and osteogenesis still requires explication. In this study, we found that FAM20B, a hexokinase essential for attaching GAG chains to the core proteins of PGs, was robustly activated in the condylar mesenchyme during TMJ development. The inactivation of Fam20b in craniofacial neural crest cells (CNCCs) dramatically reduced the synthesis and accumulation of GAG chains rather than core proteins in the condylar cartilage, which resulted in a hypoplastic condylar cartilage by severely promoting chondrocyte hypertrophy and perichondral ossification. In the condyles of Wnt1-Cre;Fam20bf/f mouse embryos, enlarged Ihh- and COL10-expressing domains indicated premature hypertrophy resulting from an attenuated IHH-PTHRP negative feedback in condylar chondrocytes, while increased osteogenic markers, canonical Wnt activity, and type-H angiogenesis verified the enhanced osteogenesis in the perichondrium. Further ex vivo investigations revealed that the loss of Fam20b decreased the domain area but increased the activity of HH signaling in the embryonic condylar mesenchyme. Moreover, the abrogation of GAG chains in heparan sulfate and chondroitin sulfate proteoglycans led to a rapid up- and then downregulation of HH signaling in condylar chondrocytes, implicating a "slow-release" manner of growth factors controlled by GAG chains. Overall, this study revealed a comprehensive role of the FAM20B-catalyzed GAG chain synthesis in the chondrogenic and osteogenic differentiation of the embryonic TMJ condyle.
Keywords: FAM20B; endochondral osteogenesis; glycosaminoglycan chain; proteoglycan; temporomandibular joint.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures



References
-
- Karamanos N.K., Piperigkou Z., Theocharis A.D., Watanabe H., Franchi M., Baud S., Brezillon S., Gotte M., Passi A., Vigetti D., et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev. 2018;118:9152–9232. doi: 10.1021/acs.chemrev.8b00354. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

