Photodynamic Effectiveness of Copper-Iminopyridine Photosensitizers Coupled to Zinc Oxide Nanoparticles Against Klebsiella pneumoniae and the Bacterial Response to Oxidative Stress
- PMID: 40362414
- PMCID: PMC12071902
- DOI: 10.3390/ijms26094178
Photodynamic Effectiveness of Copper-Iminopyridine Photosensitizers Coupled to Zinc Oxide Nanoparticles Against Klebsiella pneumoniae and the Bacterial Response to Oxidative Stress
Abstract
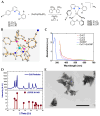
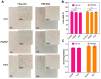
One of the most urgent threats to public health worldwide is the ongoing rise of multidrug-resistant (MDR) bacterial strains. Among the most critical pathogens are MDR-Klebsiella pneumoniae strains. The lack of new antibiotics has led to an increased need for non-antibiotic antimicrobial therapies. Photodynamic therapy (PDT) has become increasingly significant in treating MDR bacteria. PDT uses photosensitizer compounds (PS) that generate reactive oxygen species (ROS) when activated by light. These ROS produce localized oxidative stress, damaging the bacterial envelope. A downside of PDT is the limited bioavailability of PSs in vivo, which can be enhanced by conjugating them with carriers like nanoparticles (NPs). Zinc nanoparticles possess antibacterial properties, decreasing the adherence and viability of microorganisms on surfaces. The additive or synergistic effect of the combined NP-PS could improve phototherapeutic action. Therefore, this study evaluated the effectiveness of the copper(I)-based PS CuC1 compound in combination with Zinc Oxide NP, ZnONP, to inhibit the growth of both MDR and sensitive K. pneumoniae strains. The reduction in bacterial viability after exposure to a PS/NP mixture activated by 61.2 J/cm2 of blue light photodynamic treatment was assessed. The optimal PS/NP ratio was determined at 2 µg/mL of CuC1 combined with 64 µg/mL of ZnONP as the minimum effective concentration (MEC). The bacterial gene response aligned with a mechanism of photooxidative stress induced by the treatment, which damages the bacterial cell envelope. Additionally, we found that the PS/NP mixture is not harmful to mammalian cells, such as Hep-G2 and HEK-293. In conclusion, the CuC1/ZnONP combination could effectively aid in enhancing the antimicrobial treatment of infections caused by MDR bacteria.
Keywords: Klebsiella pneumoniae; ZnO nanoparticles; copper(I) complex; multi-drug resistance; photodynamic therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Inhibition of multi-drug resistant Klebsiella pneumoniae: Nanoparticles induced photoinactivation in presence of efflux pump inhibitor.Eur J Pharm Biopharm. 2020 Dec;157:165-174. doi: 10.1016/j.ejpb.2020.10.007. Epub 2020 Oct 26. Eur J Pharm Biopharm. 2020. PMID: 33122117
-
Activity of zinc oxide and zinc borate nanoparticles against resistant bacteria in an experimental lung cancer model.Daru. 2024 Jun;32(1):197-206. doi: 10.1007/s40199-024-00505-2. Epub 2024 Feb 17. Daru. 2024. PMID: 38366078 Free PMC article.
-
Synergistic effect of combined imipenem and photodynamic treatment with the cationic Ir(III) complexes to polypyridine ligand on carbapenem-resistant Klebsiella pneumoniae.Photodiagnosis Photodyn Ther. 2020 Sep;31:101882. doi: 10.1016/j.pdpdt.2020.101882. Epub 2020 Jun 18. Photodiagnosis Photodyn Ther. 2020. PMID: 32565179
-
Photodynamic treatment for multidrug-resistant Gram-negative bacteria: Perspectives for the treatment of Klebsiella pneumoniae infections.Photodiagnosis Photodyn Ther. 2019 Dec;28:256-264. doi: 10.1016/j.pdpdt.2019.08.012. Epub 2019 Sep 7. Photodiagnosis Photodyn Ther. 2019. PMID: 31505296 Review.
-
Nanoscale ZnO-based photosensitizers for photodynamic therapy.Photodiagnosis Photodyn Ther. 2020 Jun;30:101694. doi: 10.1016/j.pdpdt.2020.101694. Epub 2020 Feb 25. Photodiagnosis Photodyn Ther. 2020. PMID: 32109615 Review.
Cited by
-
Photoactive Hydrogels as Materials for Biological Applications: Preparation of Thermally Stable Photoactive Films.Gels. 2025 Aug 20;11(8):663. doi: 10.3390/gels11080663. Gels. 2025. PMID: 40868793 Free PMC article.
References
-
- Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. - DOI - PubMed
-
- WHO WHO Priority Pathogens List for R&D of New Antibiotics. [(accessed on 19 April 2021)]. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

