In Vitro Immunomodulatory Effects of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Primed with a Cannabidiol-Rich Extract
- PMID: 40362445
- PMCID: PMC12071624
- DOI: 10.3390/ijms26094208
In Vitro Immunomodulatory Effects of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Primed with a Cannabidiol-Rich Extract
Abstract
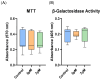
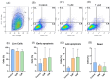
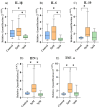
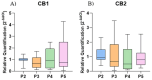
Cell-based therapy using mesenchymal stem cells (MSCs) shows promise for treating several diseases due to their anti-inflammatory and immunomodulatory properties. To enhance the therapeutic potential of MSCs, in vitro priming strategies have been explored. Cannabidiol (CBD), a non-psychoactive compound derived from cannabis, may influence MSC proliferation, differentiation, and immunomodulatory properties. This study evaluates the immunomodulatory potential of equine adipose tissue-derived MSCs (EqAT-MSCs) primed with a CBD-rich cannabis extract. EqAT-MSCs (P3) were primed with CBD concentrations of 5 µM and 7 µM for 24 h. Morphological analysis, MTT assay, β-galactosidase activity, apoptosis assays, and gene expression of interleukins IL-1β, IL-6, IL-10, interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) were conducted. Additionally, cannabinoid receptor 1 (CB1) and 2 (CB2) expression were evaluated in naïve EqAT-MSCs (P2-P5). The naïve EqAT-MSCs expressed CB1 and CB2 receptors. Priming with 5 µM significantly increased the expression of IL-10, TNF-α, and IFN-γ, while 7 µM decreased IL-1β and IL-6 expression. No significant changes were observed in other cytokines, MTT, β-galactosidase activity, or apoptosis. These findings demonstrate that naïve EqAT-MSCs express CB1 and CB2 receptors and priming with the extract modulates the expression of pro- and anti-inflammatory cytokines, highlighting its potential immunomodulatory role in EqAT-MSC-based therapies.
Keywords: cannabinoid receptors; cell therapy; cytokines; horse; phytocannabinoid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

