Molecular Mechanisms of Tumor Progression and Novel Therapeutic and Diagnostic Strategies in Mesothelioma
- PMID: 40362535
- PMCID: PMC12072309
- DOI: 10.3390/ijms26094299
Molecular Mechanisms of Tumor Progression and Novel Therapeutic and Diagnostic Strategies in Mesothelioma
Abstract
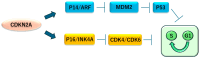
Mesothelioma is characterized by the inactivation of tumor suppressor genes, with frequent mutations in neurofibromin 2 (NF2), BRCA1-associated protein 1 (BAP1), and cyclin-dependent kinase inhibitor 2A (CDKN2A). These mutations lead to disruptions in the Hippo signaling pathway and histone methylation, thereby promoting tumor growth. NF2 mutations result in Merlin deficiency, leading to uncontrolled cell proliferation, whereas BAP1 mutations impair chromatin remodeling and hinder DNA damage repair. Emerging molecular targets in mesothelioma include mesothelin (MSLN), oxytocin receptor (OXTR), protein arginine methyltransferase (PRMT5), and carbohydrate sulfotransferase 4 (CHST4). MSLN-based therapies, such as antibody-drug conjugates and immunotoxins, have shown efficacy in clinical trials. OXTR, upregulated in mesothelioma, is correlated with poor prognosis and represents a novel therapeutic target. PRMT5 inhibition is being explored in tumors with MTAP deletions, commonly co-occurring with CDKN2A loss. CHST4 expression is associated with improved prognosis, potentially influencing tumor immunity. Immune checkpoint inhibitors targeting PD-1/PD-L1 have shown promise in some cases; however, resistance mechanisms remain a challenge. Advances in multi-omics approaches have improved our understanding of mesothelioma pathogenesis. Future research will aim to identify novel therapeutic targets and personalized treatment strategies, particularly in the context of epigenetic therapy and combination immunotherapy.
Keywords: BAP1; CHST4; FAK; Hippo signaling pathway; OXTR; immune checkpoint inhibitors; mesothelioma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Vogelzang N.J., Rusthoven J.J., Symanowski J., Denham C., Kaukel E., Ruffie P., Gatzemeier U., Boyer M., Emri S., Manegold C., et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

