Effect of Glyoxal on Plasma Membrane and Cytosolic Proteins of Erythrocytes
- PMID: 40362565
- PMCID: PMC12072774
- DOI: 10.3390/ijms26094328
Effect of Glyoxal on Plasma Membrane and Cytosolic Proteins of Erythrocytes
Abstract
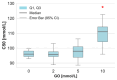
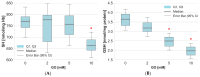
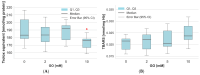
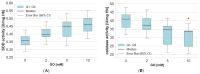
Glyoxal (GO) is a reactive dicarbonyl derived endogenously from sugars and other metabolic reactions within cells. Numerous exogenous sources of this compound include tobacco smoking, air pollution, and food processing. GO is toxic to cells mainly due to its high levels and reactivity towards proteins, lipids, and nucleic acids. We speculate that glyoxal could be involved in erythrocyte protein damage and lead to cell dysfunction. The osmotic fragility and level of amino and carbonyl groups of membrane proteins of erythrocytes incubated for 24 h with GO were identified. The amount of thiol, amino, and carbonyl groups was also measured in hemolysate proteins after erythrocyte treatment with GO. In hemolysate, the level of glutathione, non-enzymatic antioxidant capacity (NEAC), TBARS, and activity of antioxidant enzymes was also determined. The study's results indicated that GO increases erythrocyte osmotic sensitivity, alters the levels of glutathione and free functional groups in hemolysate proteins, and modifies the activity of antioxidant enzymes. Our findings indicate that GO is a highly toxic compound to human erythrocytes. Glyoxal at concentrations above 5 mM can cause functional changes in erythrocyte proteins and disrupt the oxidoreductive balance in cells.
Keywords: antioxidant enzymes; erythrocyte; glutathione; glyoxal; hemoglobin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Xie M.-Z., Guo C., Dong J.-Q., Zhang J., Sun K.-T., Lu G.-J., Wang L., Bo D.-Y., Jiao L.-Y., Zhao G.-A. Glyoxal damages human aortic endothelial cells by perturbing the glutathione, mitochondrial membrane potential, and mitogen-activated protein kinase pathways. BMC Cardiovasc. Disord. 2021;21:603. doi: 10.1186/s12872-021-02418-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

