In Situ synNotch-Programmed Astrocytes Sense and Attenuate Neuronal Apoptosis
- PMID: 40362583
- PMCID: PMC12072468
- DOI: 10.3390/ijms26094343
In Situ synNotch-Programmed Astrocytes Sense and Attenuate Neuronal Apoptosis
Abstract
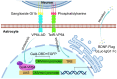
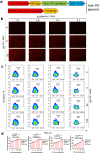
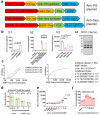
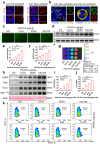
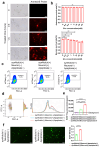
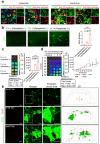
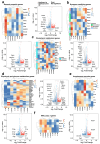
Neuronal apoptosis is an early and critical pathological hallmark of many chronic neurodegenerative diseases, often occurring silently long before the appearance of overt clinical symptoms. In this study, we engineered astrocytes utilizing a dual-biomarker recognition synNotch system (dual-synNotch). This system is designed to specifically identify neuronal apoptosis through the 'AND Gate' activation mechanism, which is triggered by the simultaneous sensing of the apoptotic signal phosphatidylserine (PS) and the neuronal signal ganglioside Gt1b. Upon detection of these neuronal apoptotic signals, the synNotch receptors are activated, inducing the expression of two key molecules: secreted Gaussia luciferase (GLuc), a highly detectable reporter that can cross the blood-brain barrier (BBB), and brain-derived neurotrophic factor (BDNF), a neuroprotective molecule that promotes neuronal survival by inhibiting apoptosis and enhancing memory and cognitive function. This engineered system effectively converts and amplifies early, imperceptible neuronal apoptotic signals into detectable outputs, enabling convenient in vitro monitoring and diagnosis. Therefore, it represents a promising strategy for the early detection and intervention of neurodegenerative diseases associated with neuronal apoptosis.
Keywords: brain-derived neurotrophic factor (BDNF); gaussia luciferase (GLuc); neuronal apoptosis; phosphatidylserine (PS); reprogrammed astrocyte; synNotch receptor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

