Assessment of Catalase Inhibition Under e-Beam Irradiation
- PMID: 40362594
- PMCID: PMC12072584
- DOI: 10.3390/ijms26094358
Assessment of Catalase Inhibition Under e-Beam Irradiation
Abstract
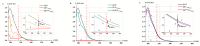
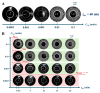
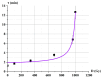
Catalase serves as a crucial component of the antioxidant defense system by catalyzing the decomposition of hydrogen peroxide into water and molecular oxygen. This study investigated the effects of 1 MeV accelerated electron irradiation on catalase activity in model solutions at doses of 100 Gy and 1000 Gy. Enzyme activity was assessed using two complementary methods: spectrophotometric analysis and the oxygen bubble method. The experimental results demonstrated dose-dependent inhibition of catalase activity, indicating that substantial radiation-induced structural modifications may occur in the enzyme molecule as a result of irradiation. To understand the relationship between the irradiation dose and the catalase inhibition, calibration curves plotting the dependencies of hydrogen peroxide decomposition rate and the delayed appearance of oxygen bubbles after adding hydrogen peroxide to catalase saline solution on the catalase concentration showed a 1.5-fold reduction in catalase activity at 100 Gy and a 40-fold decrease at 1000 Gy. Based on these findings, we propose a novel biodosimetry approach utilizing the oxygen bubble formation delay time as an express assessment tool for detecting high radiation doses absorbed by biological objects, for example, food products. The results obtained in the study have important implications for evaluating radiation effects on biological systems, in particular catalase-containing food products, offering potential applications in radiation safety monitoring and food quality control.
Keywords: accelerated electrons; biodosimetry; catalase; catalase activity; hydrogen peroxide; oxygen bubbles; radiation inhibition; radiation processing; spectrophotometry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- FAO . The State of Food Security and Nutrition in the World. FAO; Rome, Italy: IFAD; Rome, Italy: UNICEF; Rome, Italy: WFP; Rome, Italy: WHO; Rome, Italy: 2023. 316p. - DOI
-
- IAEA . Manual of Good Practice in Food Irradiation. IAEA; Vienna, Austria: 2015. pp. 1–104. (Technical Reports Series No. 481).
-
- Commission J.F.C.A. Codex General Standard for Irradiated Foods and Recommended International Code of Practice for the Operation of Radiation Facilities Used for the Treatment of Foods. Joint FAO/WHO Codex Alimentarius Commission; Rome, Italy: 1990.
-
- IAEA . Revision of the Recommended International General Standard for Irradiated Foods and of the Recommended International Code of Practice for the Operation of Radiation Facilities Used for the Treatment of Foods. Food and Agriculture Organization of the United Nations; Rome, Italy: 1981.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

