Intraplantar β-Caryophyllene Alleviates Pain and Inflammation in STZ-Induced Diabetic Peripheral Neuropathy via CB2 Receptor Activation
- PMID: 40362667
- PMCID: PMC12072555
- DOI: 10.3390/ijms26094430
Intraplantar β-Caryophyllene Alleviates Pain and Inflammation in STZ-Induced Diabetic Peripheral Neuropathy via CB2 Receptor Activation
Abstract
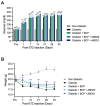
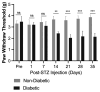
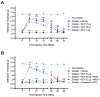
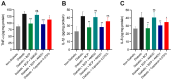
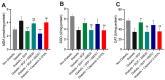
Diabetic peripheral neuropathy (DPN) is a debilitating complication of diabetes, characterized by mechanical allodynia, neuroinflammation, and oxidative stress. Current treatments offer limited efficacy and are often associated with systemic side effects. Emerging evidence suggests that activation of cannabinoid receptor type 2 (CB2) may represent a promising target for managing neuropathic pain and inflammation. This study investigates the therapeutic potential of intraplantar β-Caryophyllene (BCP), a selective CB2 receptor agonist, administered as a topical intervention in a streptozotocin (STZ)-induced DPN mouse model. Hyperglycemia was induced by STZ injections, and diabetic mice received intraplantar BCP (9, 18, or 27 µg) daily for 21 days. Mechanical allodynia was assessed using von Frey filaments, and levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) and oxidative stress markers (MDA, SOD, CAT) were quantified in hind paw tissues. BCP dose-dependently alleviated STZ-induced mechanical allodynia, with the 27 µg dose producing the most pronounced effect (p < 0.001). The anti-allodynic effects of BCP were mediated through CB2 receptor activation, confirmed by reversal with the CB2 antagonist AM630 (p < 0.001), while the CB1 antagonist AM251 had no significant impact. In addition, BCP significantly reduced pro-inflammatory cytokines (p < 0.01) and oxidative stress markers (p < 0.001) while restoring antioxidant enzyme activities (p < 0.05). A control group treated with a clinically available topical analgesic cream containing capsaicin 0.075% exhibited limited efficacy. These findings position topical BCP administration as a novel therapeutic strategy for DPN, offering sustained pain relief and modulation of neuroinflammatory and oxidative pathways with minimal systemic exposure. Further clinical studies are warranted to validate its potential for translation into therapeutic practice.
Keywords: STZ-induced neuropathy; cannabinoid receptors 2; diabetic peripheral neuropathy; β-Caryophyllene.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

References
-
- Owolabi L.F., Alghamdi M., Adamu B., Taura M.G., Jibo A., Almansour M., Alaklabi S.N., Alghamdi M.A., Alotaibi Y.A., Imam I.A., et al. Magnitude of Diabetic Peripheral Neuropathy in Saudi Arabia: A Systematic Review and Meta-Analysis. BMC Endocr. Disord. 2022;22:266. doi: 10.1186/s12902-022-01167-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

